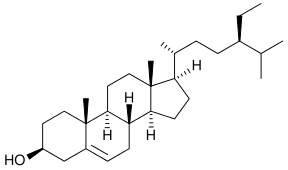
beta-Sitosterol
 | |
| Names | |
|---|---|
| IUPAC name
17-(5-Ethyl-6-methylheptan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol | |
| Other names
22,23-Dihydrostigmasterol, Stigmast-5-en-3-ol, β-Sitosterin | |
| Identifiers | |
| 83-46-5 | |
| ChEBI | CHEBI:27693 |
| ChEMBL | ChEMBL221542 |
| ChemSpider | 192962 |
| |
| Jmol-3D images | Image |
| PubChem | 222284 |
| |
| UNII | S347WMO6M4 |
| Properties | |
| Molecular formula |
C29H50O |
| Molar mass | 414.71 g·mol−1 |
| Melting point | 136 to 140 °C (277 to 284 °F; 409 to 413 K)[1] |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
β-Sitosterol is one of several phytosterols (plant sterols) with chemical structures similar to that of cholesterol. Sitosterols are white, waxy powders with a characteristic odor. They are hydrophobic and soluble in alcohols.
Natural occurrences
It is widely distributed in the plant kingdom and found in Nigella sativa, Serenoa repens (saw palmetto), Pygeum africanum, sea-buckthorn, wolfberries, Mirabilis jalapa,[2] Cannabis sativa, Urtica dioica,[3] and Wrightia tinctoria.
Occurrences in food
It is found in pecans, avocados, Cucurbita pepo (pumpkin seeds), cashew fruit, rice bran, wheat germ, corn oils, soybeans and dandelion coffee.
Human research
β-sitosterol is being studied for its potential to reduce benign prostatic hyperplasia (BPH)[4][5] and blood cholesterol levels.[6]
Side effects
High levels of β-sitosterol concentrations in blood have been correlated with increased severity of heart disease in men having previously suffered from heart attacks.[7]
Genetic disorder
While plant sterols are usually beneficial, there is a rare autosomal recessive genetic disorder phytosterolemia which causes over-absorption of phytosterols.[8]
Precursor of anabolic steroid boldenone
Being a steroid, β-sitosterol is precursor of anabolic steroid boldenone. Boldenone undecylenate is commonly used in veterinary medicine to induce growth in cattle but it is also one of the most commonly abused anabolic steroids in sports. This led to suspicion that some athletes testing positive on boldenone undecylenate didn't actually abuse the hormone itself but consumed food rich in β-sitosterol.[9][10][11]
Chemistry
Chemical engineering
The use of sitosterol as a chemical intermediate was for many years limited due to the lack of a chemical point of attack on the side-chain that would permit its removal. Extensive efforts on the part of many laboratories eventually led to the discovery of a pseudomonas microbe that efficiently effected that transformation. Fermentation digests the entire aliphatic side-chain at carbon 17 to afford a mixture of 17-keto products including dehydroepiandrosterone.[12]
Synthesis
Total synthesis of β-sitosterol has not been achieved. However, β-sitosterol has been synthesized from stigmasterol 1, which involves a specific hydrogenation of the side-chain of stigmasterol (See Figure Below). The first step in the synthesis forms stigmasterol tosylate 2 from stigmasterol 1 (95% purity) using p-TsCl, DMAP, and pyridine (90% yield). The tosylate 2 then undergoes solvolysis as it is treated with pyridine and anhydrous MeOH to give a 5:1 ratio of i-stigmasterol methyl ether 3 (74% yield) to stigmasterol methyl ether 4, which is subsequently removed by chromatography. The hydrogenation step of a previously proposed synthesis involved the catalyst Pd/C and the solvent ethyl acetate. However, due to isomerisation during hydrolysis, other catalysts, such as PtO2, and solvents, such as ethanol, were tested. There was little change with the use of a different catalyst. Ethanol, however, prevented isomerisation and the formation of the unidentified impurity to give compound 5. The last step of the synthesis is deprotection of the β-ring double bond of 5 with p-TsOH, aqueous dioxane, and heat (80 °C) to yield β-sitosterol 6. The cumulative yield for the final two steps was 55%, and the total yield for the synthesis was 37%.[13]

Biosynthesis
The regulation of the biosynthesis of both sterols and some specific lipids occurs during membrane biogenesis.[14] Through 13C-labeling patterns, it has been determined that both the mevalonate and deoxyxylulose pathways are involved in the formation of β-sitosterol.[15] The precise mechanism of β-sitosterol formation varies according to the organism, but is generally found to come from cycloartenol.[16]
The biosynthesis of cycloartenol begins as one molecule of isopentenyl diphosphate (IPP) and two molecules of dimethylallyl diphosphate (DMAPP) form farnesyl diphosphate (FPP). Two molecules of FPP are then joined tail-to-tail to yield squalene, a triterpene. Squalene, through a cyclization reaction with 2,3-oxidosqualene 6 as an intermediate forms cycloartenol.
The biosynthesis of β-sitosterol from cycloartenol is summarized below.

The double bond of cycloartenol (compound 7 in diagram) is methylated by SAM to give a carbocation that undergoes a hydride shift and loses a proton to yield a compound with a methylene side-chain. Both of these steps are catalyzed by sterol C-24 methyltransferase (Step E1 in diagram). Compound 8 is then catalyzed by sterol C-4 demethylase (E2) and loses a methyl group to produce cycloeucalenol. Subsequent to this, the cyclopropane ring is opened with cycloeucalenol cycloisomerase (E3) to form 10. Compound 10 loses a methyl group and undergoes an allylic isomerization to form Gramisterol 11. This step is catalyzed by sterol C-14 demethylase (E4), sterol Δ14-reductase (E5), and sterol Δ8-Δ7-isomerase (E6). The last methyl group is removed by sterol demethylase (E7) to form episterol 12. Episterol 12 is methylated by SAM to produce a second carbocation, which loses a proton to yield 13. This step is catalyzed by 24-methylenesterol C-methyltransferase (E8). Compound 13 now undergoes reduction by NADPH and modifications in the β-ring to form β-sitosterol.
See also
- Charantin, a β-sitosteryl glucoside found in the bitter melon plant.
References
- ↑ Oja, Vahur; Chen, Xu; Hajaligol, Mohammad R.; Chan, W. Geoffrey (2009). "Sublimation Thermodynamic Parameters for Cholesterol, Ergosterol, β-Sitosterol, and Stigmasterol". Journal of Chemical & Engineering Data 54 (3): 730–734. doi:10.1021/je800395m.
- ↑ Siddiqui S., Siddiqui B.S., Adil Q. and Begum S. (1990). "Constituents of Mirabilis jalapa". Fitoterapia 61 (5): 471.
- ↑ Kopyt’Ko, Ya. F.; Lapinskaya, E. S.; Sokol’Skaya, T. A. (January 2012). "Application, chemical composition, and standardization of nettle raw material and related drugs (Review)". Pharmaceutical Chemistry Journal 45 (10): 622. doi:10.1007/s11094-012-0690-7. Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 45, No. 10, pp. 32 – 41, October, 2011.
- ↑ Wilt, T; Ishani, A; MacDonald, R; Stark, G; Mulrow, C; Lau, J (2000). "Beta-sitosterols for benign prostatic hyperplasia". The Cochrane Library (2): CD001043. doi:10.1002/14651858.CD001043. PMID 10796740.
- ↑ Kim, T. H.; Lim, H. J.; Kim, M. S.; Lee, M. S. (2012). "Dietary supplements for benign prostatic hyperplasia: An overview of systematic reviews". Maturitas 73 (3): 180–5. doi:10.1016/j.maturitas.2012.07.007. PMID 22883375.
- ↑ Rudkowska I, AbuMweis SS, Nicolle C, Jones PJ (2008). "Cholesterol-lowering efficacy of plant sterols in low-fat yogurt consumed as a snack or with a meal". J Am Coll Nutr 27 (5): 588–95. doi:10.1080/07315724.2008.10719742. PMID 18845709.
- ↑ Assmann G, Cullen P, Erbey J, Ramey DR, Kannenberg F, Schulte H (January 2006). "Plasma sitosterol elevations are associated with an increased incidence of coronary events in men: results of a nested case-control analysis of the Prospective Cardiovascular Münster (PROCAM) study". Nutrition, Metabolism, and Cardiovascular Diseases : NMCD 16 (1): 13–21. doi:10.1016/j.numecd.2005.04.001. PMID 16399487.
- ↑ Patel Manoj D., Thompson Paul D. (2006). "Phytosterols and Vascular Disease". Atherosclerosis 186 (1): 12–19. doi:10.1016/j.atherosclerosis.2005.10.026. PMID 16325823.
- ↑ G. Gallina, G. Ferretti, R. Merlanti, C. Civitareale, F. Capolongo, R. Draisci and C. Montesissa (2007). "Boldenone, Boldione, and Milk Replacers in the Diet of Veal Calves: The Effects of Phytosterol Content on the Urinary Excretion of Boldenone Metabolites". J. Agric. Food Chem. 20 (20): 8275–8283. doi:10.1021/jf071097c. PMID 17844992.
- ↑ Ros MM, Sterk SS, Verhagen H, Stalenhoef AF, de Jong N. (2007). "Phytosterol consumption and the anabolic steroid boldenone in humans: a hypothesis piloted". Food Addit Contam. 24 (7): 679–84. doi:10.1080/02652030701216727. PMID 17613052.
- ↑ R. Draisci, R. Merlanti, G. Ferretti, L. Fantozzi, C. Ferranti, F. Capolongo, S. Segato, C. Montesissa (2007). "Excretion profile of boldenone in urine of veal calves fed two different milk replacers". Analytica Chimica Acta 586 (1–2): 171–176. doi:10.1016/j.aca.2007.01.026. PMID 17386709.
- ↑ Lenz, G. R.; Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed., Wiley Interscience, London, 1983, Vol. 21, 645.
- ↑ McCarthy, FO; Chopra, J; Ford, A; Hogan, SA; Kerry, JP; O'Brien, NM; Ryan, E; Maguire, AR (2005). "Synthesis, isolation and characterisation of beta-sitosterol and beta-sitosterol oxide derivatives". Organic & biomolecular chemistry 3 (16): 3059–65. doi:10.1039/b505069c. PMID 16186940.
- ↑ Hartmann, Marie-Andrée (2003). "Lipid Metabolism and Membrane Biogenesis". Topics in Current Genetics 6. p. 183. doi:10.1007/978-3-540-40999-1_6. ISBN 978-3-540-20752-8.
|chapter=ignored (help) - ↑ De-Eknamkul W., Potduang B. (2003). "Biosynthesis of β-Sitosterol and Stigmasterol in Croton sublyratus Proceeds Via a Mixed Origin of Isoprene Units". Phytochemistry 62 (3): 389–398. doi:10.1016/S0031-9422(02)00555-1. PMID 12620352.
- ↑ Dewick, P. M. Medicinal Natural Products: A Biosynthetic Approach. 3 ed.; John Wiley & Sons Ltd.: United Kingdom cyclization, 2009; p 539.
| ||||||