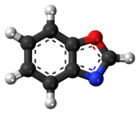
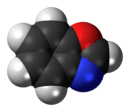
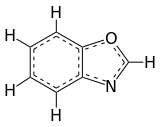
Benzoxazole
 | |||
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
benzoxazole | |||
| Other names
1-Oxa-3-aza-1H-indene | |||
| Identifiers | |||
| 273-53-0 | |||
| ChEBI | CHEBI:38814 | ||
| ChEMBL | ChEMBL451894 | ||
| ChemSpider | 8873 | ||
| |||
| Jmol-3D images | Image | ||
| PubChem | 9228 | ||
| |||
| Properties | |||
| Molecular formula |
C7H5NO | ||
| Molar mass | 119.12 g·mol−1 | ||
| Appearance | white to light yellow solid | ||
| Melting point | 27 °C (81 °F; 300 K) | ||
| Boiling point | 182 °C (360 °F; 455 K) | ||
| insol. | |||
| Hazards | |||
| Flash point | 58 °C (136 °F; 331 K) | ||
| Related compounds | |||
| Related compounds |
oxazole indole benzofuran | ||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| | |||
| Infobox references | |||
Benzoxazole is an aromatic organic compound with a molecular formula C7H5NO, a benzene-fused oxazole ring structure, and an odor similar to pyridine.[1][2] Benzoxazole is used primarily in industry and research, and has no household use.
Being a heterocyclic compound, benzoxazole finds use in research as a starting material for the synthesis of larger, usually bioactive structures. It is found within the chemical structures of pharmaceutical drugs such as flunoxaprofen.
Its aromaticity makes it relatively stable, although as a heterocycle, it has reactive sites which allow for functionalization.
See also
- Benzisoxazole, an analog with the nitrogen atom in position 2.
- Benzimidazole, an analog with the oxygen replaced by a nitrogen.
- Benzothiazole, an analog with the oxygen replaced by a sulfur.
- Benzopyrrole or indole, an analog without the oxygen atom.
- Benzofuran, an analog without the nitrogen atom.
- Benzoxazoline, which has one double bond reduced.
- Benzoxazolidinedione, which has both double bonds reduced.
- Benzoxazolidine, which has both double bonds reduced.
- Benzoxazolidinone
- Simple aromatic rings
References
- ↑ Katritzky, A. R.; Pozharskii, A. F. (2000). Handbook of Heterocyclic Chemistry (2nd ed.). Academic Press. ISBN 0080429882.
- ↑ Clayden, J.; Greeves, N.; Warren, S.; Wothers, P. (2001). Organic Chemistry. Oxford, Oxfordshire: Oxford University Press. ISBN 0-19-850346-6.