Benzoin condensation
The benzoin condensation is a reaction (often called a condensation reaction, for historical reasons) between two aromatic aldehydes, particularly benzaldehyde. The reaction is catalyzed by a nucleophile such as the cyanide anion or an N-heterocyclic carbene. The reaction product is an aromatic acyloin with benzoin as the parent compound.[1] An early version of the reaction was developed in 1832 by Justus von Liebig and Friedrich Woehler during their research on bitter almond oil.[2] The catalytic version of the reaction was developed by Nikolay Zinin in the late 1830s,[3][4] and the reaction mechanism for this organic reaction was proposed in 1903 by A. J. Lapworth.[5]
Reaction mechanism
In the first step in this reaction, the cyanide anion (as sodium cyanide) reacts with the aldehyde in a nucleophilic addition. Rearrangement of the intermediate results in polarity reversal of the carbonyl group, which then adds to the second carbonyl group in a second nucleophilic addition. Proton transfer and elimination of the cyanide ion affords benzoin as the product. This is a reversible reaction.
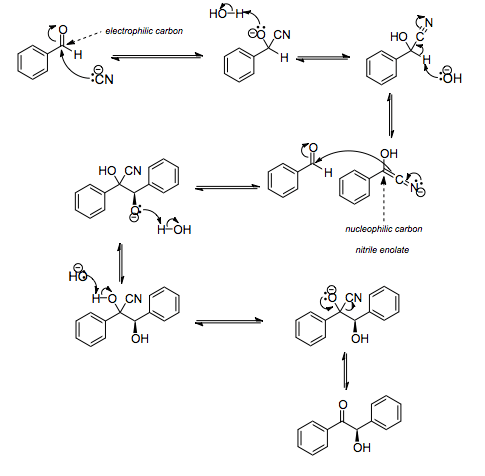
The cyanide ion serves three different purposes in the course of this reaction. It acts as a nucleophile, facilitates proton abstraction, and is also the leaving group in the final step. The benzoin condensation is in effect a dimerization and not a condensation because a small molecule like water is not released in this reaction. For this reason the reaction is also called a benzoin addition. In this reaction, the two aldehydes serve different purposes; one aldehyde donates a proton and one aldehyde accepts a proton. 4-Dimethylaminobenzaldehyde is an efficient proton donor while benzaldehyde is both a proton acceptor and donor. In this way it is possible to synthesise mixed benzoins, i.e. products with different groups on each half of the product.
Scope
The reaction can be extended to aliphatic aldehydes with base catalysis in the presence of thiazolium salts; the reaction mechanism is essentially the same. These compounds are important in the synthesis of heterocyclic compounds. The addition is also possible with enones; for instance methyl vinyl ketone is a reagent in the Stetter reaction.
In biochemistry, the coenzyme thiamine is responsible for biosynthesis of acyloin-like compounds. This coenzyme also contains a thiazolium moiety, which on deprotonation becomes a nucleophilic carbene.
In one study, a custom-designed N-heterocyclic carbene (NHC, the framework is related to thiazolium salts) was found to facilitate an enantioselective intramolecular benzoin condensation (Scheme 2).[6]

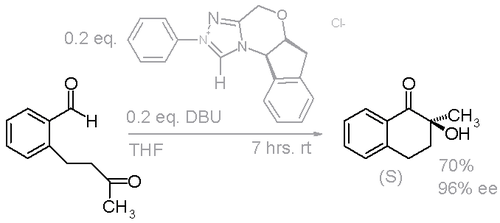
This finding was confirmed in another study with a slightly modified NHC using DBU as the base instead of potassium tert-butoxide (Scheme 3).[7]
References
- ↑ Roger Adams and C. S. Marvel (1941). "Benzoin". Org. Synth.; Coll. Vol. 1, p. 94
- ↑ Wöhler, Liebig; Liebig (1832). "Untersuchungen über das Radikal der Benzoesäure". Annalen der Pharmacie 3 (3): 249–282. doi:10.1002/jlac.18320030302.
- ↑ N. Zinin (1839). "Beiträge zur Kenntniss einiger Verbindungen aus der Benzoylreihe". Annalen der Pharmacie 31 (3): 329–332. doi:10.1002/jlac.18390310312.
- ↑ N. Zinin (1840). "Ueber einige Zersetzungsprodukte des Bittermandelöls". Annalen der Pharmacie 34 (2): 186–192. doi:10.1002/jlac.18400340205.
- ↑ Lapworth, A. (1904). "CXXII.—Reactions involving the addition of hydrogen cyanide to carbon compounds. Part II. Cyanohydrins regarded as complex acids". Journal of the Chemical Society, Transactions 85: 1206–1214. doi:10.1039/CT9048501206.
- ↑ D. Enders, O. Niemeier and T. Balensiefer (2006). "Asymmetric Intramolecular Crossed-Benzoin Reactions by N-Heterocyclic Carbene Catalysis". Angewandte Chemie International Edition 45 (9): 1463–1467. doi:10.1002/anie.200503885. PMID 16389609.
- ↑ H. Takikawa, Y. Hachisu, J. W. Bode and K. Suzuki (2006). "Catalytic Enantioselective Crossed Aldehyde-Ketone Benzoin Cyclization". Angewandte Chemie International Edition 45 (21): 3492–3494. doi:10.1002/anie.200600268. PMID 16637094.