Batesian mimicry
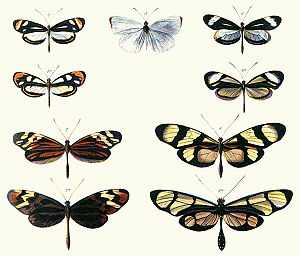
Batesian mimicry is a form of mimicry typified by a situation where a harmless species has evolved to imitate the warning signals of a harmful species directed at a common predator. It is named after the English naturalist Henry Walter Bates, after his work in the rainforests of Brazil.
Batesian mimicry is the most commonly known and widely studied of mimicry complexes, such that the word mimicry is often treated as synonymous with Batesian mimicry. There are many other forms however, some very similar in principle, others far separated. Of note, it is often contrasted with Müllerian mimicry, a form of mutually beneficial convergence between two or more harmful species. However, because the mimic may have a degree of protection itself, the distinction is not absolute. It can also be contrasted with functionally different forms of mimicry. Perhaps the sharpest contrast here is with aggressive mimicry, where a predator or parasite mimics a harmless species, avoiding detection and improving its foraging success.
The organism imitating the protected species is referred to as the mimic, while the imitated organism is known as the model. The receiver mediating indirect interactions between these two parties is variously known as the signal receiver, dupe or operator. By parasitizing the honest warning signal of the protected species, the Batesian mimic gains the same advantage, without having to go to the expense of arming themselves. The model, on the other hand, is disadvantaged, along with the dupe. If imposters appear in high numbers, positive experiences with the mimic may result in the model being treated as harmless. Additionally, in higher frequency there is a stronger selective advantage for the predator to distinguish mimic from model. For this reason, mimics are usually less numerous than models. However, some mimetic populations have evolved multiple forms (polymorphism), enabling them to mimic several different models. This affords them greater protection, a concept in evolutionary biology known as frequency dependent selection.
Batesian mimicry need not involve visual mimicry, but can employ deception of any of the senses. For example, some moths mimic the ultrasound warning signals sent by unpalatable moths to bat predators, a case of auditory Batesian mimicry. A cocktail of deceptive signals may also be used. The distinction between Batesian mimicry and crypsis is clear: the mimic is noticed, but treated as something it is not. On the other hand, camouflaged prey would often create the same effect by being invisible. While model and mimic are often from related taxa, mimicry of very distant relatives is also known. The majority of Batesian mimics are insects.
Historical background
Henry Walter Bates: (1825–1892) was an English explorer-naturalist who surveyed the Amazon Rainforest with Alfred Russel Wallace in 1848. While Wallace returned in 1852, Bates remained for over a decade. His field research included collecting almost a hundred species of butterflies from the families Ithomiinae and Heliconiinae, as well as thousands of other insects specimens. In sorting these butterflies into similar groups based on appearance, inconsistencies began to arise. Some appeared superficially similar to others, even so much so that Bates could not tell some species apart based only on wing appearance. However, closer examination of less obvious morphological characters seemed to show that they were not even closely related. Shortly after his return to England he read a paper on his theory of mimicry at a meeting of the Linnean Society of London on the 21st of November 1861, which was then published in 1862 as 'Contributions to an Insect Fauna of the Amazon Valley' in the Transactions of the Linnaean Society.[1] He elaborated on his experiences further in The Naturalist on the River Amazons.[2] These new findings and speculations stimulated long lasting discussion and controversy, not limited to the scientific realm.
Bates put forward the hypothesis that the close resemblance between unrelated species was an antipredator adaptation. He noted that some species showed very striking coloration, and flew in a leisurely manner, almost as if taunting predators to eat them. He reasoned that these butterflies were unpalatable to birds and other insectivores, and were thus avoided by them. He extended this logic to forms that closely resembled such protected species, mimicking their warning coloration but not their toxicity.
This naturalistic explanation fitted well with the recent account of evolution by Alfred Russel Wallace and Charles Darwin, as outlined in his famous 1859 book The Origin of Species. Because this Darwinian explanation required no supernatural forces, it met with considerable criticism from anti-evolutionists, both in academic circles and in the broader social realm. The term mimicry had only been used for people until about 1850, when the word took on a new life in its application to other life forms such as plants and animals. Just as Darwin was the first to put forward a comprehensive explanation for evolution, Bates was the first to elucidate this form of mimicry, and he is thus honored with the term Batesian mimicry. Although other forms have been discovered even in recent times, Batesian mimicry is one of the most commonly occurring and well understood. To many, the word Batesian mimicry and mimicry are treated as the same thing, however Bates described several kinds himself.[3]
Aposematism

Most living things have at least one predator, with which they are in a constant evolutionary arms race to develop protective adaptations. Some organisms have evolved to make detection less likely; this is known as camouflage. Other organisms are not profitable for potential predators even if they do locate them. Still others however are harmful even if the predator can eat them, for example many plants and fungi contain deadly toxins and other chemicals, while certain snakes, wasps, and other animals are able to poison, injure, or otherwise harm many of the predators who would otherwise eat them. Such prey often send clear warning signals to their attackers, such as strong odors, bright colours and warning sounds.
Use of such messages is known as aposematism. Aposematic prey need not display such signals all the time. It may be energetically costly for them to do so, and even if it is not, they may have other predators that can tolerate their defenses. In fact, even if all their predators will avoid them if adequately warned, there are still those predators that have not yet learned that they are dangerous. Short of instinctive programming to avoid the aposematic organism (which is seen occasionally), it is unlikely that any potential prey will be prepared to sacrificially educate its predator. Thus, a combination of camouflage and its antithesis, aposematism, often occur.
However, once a predator has learned from harsh experience not to go after such prey, it will be likely to avoid anything that looks even remotely similar if it can. It is in this fashion that Batesian mimics have evolved. It is often misunderstood that such a mimic is somehow responsible itself for its mimetic characteristics. This is quite a serious misunderstanding, however. It is the duped predator that does the selecting, choosing to avoid those prey which look most like the aposematic model. In this way, the signal receiver directs the evolution of the mimic toward closer and closer similarity to the model.
Classification and comparisons
Batesian mimicry is a case of protective or defensive mimicry, where the mimic does best by avoiding confrontations with the signal receiver. It is a disjunct system, which means that all three parties are from different species.[5] Batesian mimicry stands in contrast to other forms such as aggressive mimicry, where the mimic profits from interactions with the signal receiver. One such case of this is in fireflies, where females of one species mimic the mating signals of another species, deceiving males to come close enough for them to eat. Mimicry need not involve a predator at all though. Such is the case in dispersal mimicry, where the mimic once again benefits from the encounter. For instance, some fungi have their spores dispersed by insects by smelling like carrion. In protective mimicry, the meeting between mimic and dupe is not such a fortuitous occasion for the mimic, and the signals it mimics tend to lower the probability of such an encounter.
One case somewhat similar to Batesian mimicry is that of mimetic weeds, which imitate agricultural crops. Once again, this is the result of the signal receiver's action, not a cunning ploy of the mimic. In weed, or Vavilovian mimicry, the weed does not profit from encounters with man or his winnowing machinery; at best the weed is left, at worst it is destroyed. Vavilovian mimicry is not a case of Batesian mimicry, however, because man and crop are not enemies. Indeed, the crops derive their protection from insects, weeds, and competition with other plants from their growers.
Another analogous case within a single species has been termed Browerian mimicry[3] (after Lincoln P. Brower and Jane Van Zandt Brower[6][7]). This is a case of bipolar (only two species involved) automimicry;[5] the model is same species as its mimic. Equivalent to Batesian mimicry within a single species, it occurs when there is a palatability spectrum within a population of harmful prey. For example, Monarch Butterflies (Danaus plexippus) feed on milkweed species of varying toxicity. Some larvae will feed on more toxic plants, and store these toxins within themselves, while others will not. The more palatable caterpillars will thus profit from those that ingest high levels of toxic substances, just as other butterfly species benefit from mimicry of Monarchs.
Comparison with Müllerian mimicry
Batesian mimicry belongs to a subclass of protective mimicry which can be called aposematic mimicry - the mimicry of an aposematic, protected species. Another important form of protective mimicry is Müllerian mimicry, named after the naturalist Fritz Müller. Müllerian mimicry is similar to Batesian mimicry in some respects, but quite opposite in others. In Müllerian mimicry the model is an aposematic prey as well, but the mimic itself is also aposematic, with its own true protection. Such cases troubled Bates, which he could offer no explanation for. If the mimic was protected already, what did it have to gain by copying another organism?
Müller came up with an explanation for this puzzle in 1878. Unlike in Batesian mimicry, the model is not being pirated by the mimic. In fact, the key here is that the model actually benefits from being mimicked, because it can share the troublesome burden of enlightening the predator of its harmful properties. In this cooperative enterprise, both parties benefit. It could thus be classified as a form of mutualism, an ecological relationship where two species gain mutual advantage from a biological interaction; in this case via the signal receiver.
In this account, it has been assumed that one species acts as a mimic and the other as a model. But which species should be designated each part? If two aposematic species that encounter the predator in equal number equally often come to mimic each other, it becomes completely arbitrary to call one a mimic and another a model. In fact, both can be said to be comimics, as the role of mimic and model is shared by both. Each species gains from the negative experiences of their common predator with the other. Another problem is that the predator isn't actually deceived regarding the harmful properties of the 'mimic', as both species are truly harmful. For these two reasons, some have suggested Müllerian mimicry is not mimicry at all, and have proposed terms such as Müllerian resemblance or Müllerian convergence. Looked at in another light however, it can still be seen as a form of deception in that the signal receiver treats the species it has not had an unpleasant experience with as if it were the model. This is a case of mistaken identity, although one that benefits the predator. Whether treated as mimicry or not, Müllerian convergences certainly break many of the assumptions that normally apply to mimicry complexes, and are quite the opposite of Batesian mimicry.
Occurrence
Imperfect Batesian mimicry
One of the most studied aspects of Batesian mimicry centers around the existence of imperfect or poor mimics, which do not exactly resemble their models. There are multiple theories behind why there are poor mimics, including that they are simply evolving toward perfection;[9] that they may gain advantage from resembling multiple mimics at once;[10] that humans may evaluate mimics in different ways than the actual predators;[11] that mimics may confuse predators by resembling both model and nonmimic at the same time (satiric mimicry);[12] that kin selection may enforce poor mimicry;[13] that mimics may not gain enough by being perfect mimics to make it worth giving up other advantages like thermoregulation, or cryptic coloration.[14] Another cause for imperfect mimicry may be due to limits in predator cognition. In a study conducted by Kikuchi and Pfennig, evidence was found supporting the hypothesis that only certain traits may be required to deceive predators.[15] Tests conducted on the sympatry/allopatry border of the mimic, Lampropeltis elapsoides, and the model, Micrurus fulvius, yielded that color proportions in these snakes were important in deceiving predators, but not the order of the colored rings.[15] Thus, mimics may be able to receive complete protection from mammalian predators that lack color vision by resembling the model in different shades of gray, with the same proportions.[15] More than likely it is a combination of many of these factors under different circumstances.
Acoustic mimicry
Though visual mimicry has been extensively researched, acoustic mimicry is also known, and occurs in a variety of species. Predators may identify their prey by sound as well as sight, and mimics have evolved that play tricks on the hearing of those that would eat them.
One example is the use of ultrasonic resistance. Bats rely heavily on echolocation to detect their prey, such that their auditory system might well be equivalent both in importance and perceptual nature to the human visual system.[16] Some potential prey are unpalatable to bats however, and produce an ultrasonic aposematic signal, the auditory equivalent of warning coloration. In response to echolocating red and big brown bats, tiger moths produce warning sounds. Bats learn to avoid the harmful moths, but due to their association of the warning signal with danger, they similarly avoid other species that produce such warning sounds as well. Results like these indicate acoustic mimicry complexes, both Batesian and Mullerian, may be widespread in the auditory world.[17]
See also
- Phylogenetics of mimicry
- Papilio dardanus (females mimic multiple model species)
References
- ↑ Bates, H. W. (1861). "Contributions to an insect fauna of the Amazon valley. Lepidoptera: Heliconidae". Transactions of the Linnean Society 23 (3): 495–566. doi:10.1111/j.1096-3642.1860.tb00146.x.; Reprint: Bates, Henry Walter (1981). "Contributions to an insect fauna of the Amazon valley (Lepidoptera: Heliconidae)". Biological Journal of the Linnean Society 16 (1): 41–54. doi:10.1111/j.1095-8312.1981.tb01842.x.
- ↑ Bates H. W. 1863.
- The Naturalist on the River Amazons at Project Gutenberg Murray, London.
- ↑ 3.0 3.1 Pasteur, Georges (1982). "A classificatory review of mimicry systems". Annual Review of Ecology and Systematics 13: 169–199. doi:10.1146/annurev.es.13.110182.001125.
- ↑ Ritland, D.; L. P. Brower (1991). "The viceroy butterfly is not a Batesian mimic". Nature 350 (6318): 497–498. Bibcode:1991Natur.350..497R. doi:10.1038/350497a0. Retrieved 2008-02-23.
Viceroys are as unpalatable as monarchs, and significantly more unpalatable than queens from representative Florida populations.
- ↑ 5.0 5.1 Vane-Wright, R. I. (1976). "A unified classification of mimetic resemblances". Biological Journal of the Linnean Society 8: 25–56. doi:10.1111/j.1095-8312.1976.tb00240.x.
- ↑ Brower, L. P. (1970) Plant poisons in a terrestrial food chain and implications for mimicry theory. In K. L. Chambers (ed) Biochemical Coevolution Corvallis, OR: Oregon State Univ. pp. 69-82.
- ↑ Brower, L. P.; Van Brower, J. V. Z.; Corvino, J. M. (1967). "Plant poisons in a terrestrial food chain". Proceedings of the National Academy of Sciences of the United States of America 57 (4): 893–98. Bibcode:1967PNAS...57..893B. doi:10.1073/pnas.57.4.893. PMC 224631. PMID 5231352.
- ↑ Meyer, A (2006). "Repeating Patterns of Mimicry". PLoS Biol 4 (10): e341. doi:10.1371/journal.pbio.0040341. PMC 1617347. PMID 17048984.
- ↑ Holloway, G.; Gilbert, F.; Brandt, A. (2002). "The relationship between mimetic imperfection and phenotypic variation in insect colour patterns". Proceedings of the Royal Society B 269 (1489): 411–416. doi:10.1098/rspb.2001.1885.
- ↑ Edmunds, M. (2000). "Why are there good and poor mimics?". Biological Journal of the Linnean Society 70 (3): 459–466. doi:10.1111/j.1095-8312.2000.tb01234.x.
- ↑ Dittrich, W.; Gilbert, F.; Green, P.; McGregor, P.; Grewcock, D. (1993). "Imperfect mimicry – a pigeons perspective". Proceedings of the Royal Society B 251 (1332): 195–200. doi:10.1098/rspb.1993.0029.
- ↑ Howse, P. E.; Allen, J. A. (1994). "Satyric mimicry – the evolution of apparent imperfection". Proceedings of the Royal Society B 257 (1349): 111–114. doi:10.1098/rspb.1994.0102.
- ↑ Johnstone, R. A. (2002). "The evolution of inaccurate mimics". Nature 418 (6897): 524–526. Bibcode:2002Natur.418..524J. doi:10.1038/nature00845. PMID 12152077.
- ↑ Harper, GR; Pfennig, DW (2007). "Mimicry on the edge: Why do mimics vary in resemblance to their model in different parts of their geographical range?". Proceedings of the Royal Society B 274 (1621): 1955–61. doi:10.1098/rspb.2007.0558. PMC 2275182. PMID 17567563.
- ↑ 15.0 15.1 15.2 Kikuchi, David W.; Pfennig, David W. (December 2010). "Predator Cognition Permits Imperfect Coral Snake Mimicry". The American Naturalist 176 (6): 830–834. doi:10.1086/657041. PMID 20950143.
- ↑ Dawkins, Richard (1986). The Blind Watchmaker. New York: W. W. Norton & Company, Inc. ISBN 0-393-31570-3.
- ↑ Barber, J. R.; Conner, W. E. (2007). "Acoustic mimicry in a predator prey interaction". Proceedings of the National Academy of Sciences of the United States of America 104 (22): 9331–9334. Bibcode:2007PNAS..104.9331B. doi:10.1073/pnas.0703627104. PMC 1890494. PMID 17517637.
Further reading
- Bates, H. W. (1862). "Contributions to an insect fauna of the Amazon valley. Lepidoptera: Heliconidæ". Transactions of the Linnean Society of London 23 (3): 495–566. doi:10.1111/j.1096-3642.1860.tb00146.x. Original paper by Bates. A shortened version is reprinted in Biological Journal of the Linnean Society (1981) 16: 41-54.
- Cott, H.B. (1940) Adaptive Coloration in Animals. Methuen and Co, Ltd., London ISBN 0-416-30050-2 Provides many examples of Batesian Mimicry
- Evans, M. A. (1965). "Mimicry and the Darwinian Heritage". Journal of the History of Ideas 26 (2): 211–220. doi:10.2307/2708228. For a historical perspective.
- Wickler, W. (1968) Mimicry in Plants and Animals (Translated from the German) McGraw-Hill, New York. ISBN 0-07-070100-8 Especially the first two chapters.
- Edmunds, M. 1974. Defence in Animals: A Survey of Anti-Predator Defences. Harlow, Essex & NY: Longman 357 p. ISBN 0-582-44132-3 Chapter 4 discusses this phenomenon.
- Pasteur, Georges (1982). "A classificatory review of mimicry systems". Annual Review of Ecology and Systematics 13: 169–199. doi:10.1146/annurev.es.13.110182.001125. A detailed discussion of the different forms of mimicry.
- Ruxton, G. D.; Speed, M. P.; Sherratt, T. N. (2004). Avoiding Attack. The Evolutionary Ecology of Crypsis, Warning Signals and Mimicry. Oxford: Oxford University Press. ISBN 0-19-852860-4 Chapter 10 and 11 provide an up to date synopsis
External links
- Review of Contributions to an insect fauna of the Amazon valley by Charles Darwin The Complete Works of Charles Darwin Online.
- Biographical sketch of Bates, with picture
| Wikisource has original text related to this article: |
| ||||||||||||
