Baricitinib
 | |
| Systematic (IUPAC) name | |
|---|---|
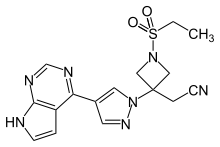
| 2-[1-ethylsulfonyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrazol-1-yl]azetidin-3-yl]acetonitrile | |
| Clinical data | |
| |
| Identifiers | |
| 1187594-09-7 | |
| None | |
| PubChem | CID 44205240 |
| ChemSpider | 26373084 |
| ChEMBL | CHEMBL2105759 |
| PDB ligand ID | 3JW (PDBe, RCSB PDB) |
| Chemical data | |
| Formula | C16H17N7O2S |
| 371.42 g/mol | |
|
SMILES
| |
Baricitinib (formerly INCB28050, LY3009104)[1] is an oral JAK1 and JAK2 inhibitor.
Baricitinib is in Phase III development by Eli Lilly and Incyte as a potential treatment for rheumatoid arthritis.[2] It is in Phase II development as a potential treatment for psoriasis and diabetic nephropathy. The related compound in JAK inhibitor is Tofacitinib, currently approved for the treatment of rheumatoid arthritis (RA) in the United States.
References
- ↑ "Baricitinib" (PDF). Statement on a nonproprietary name adopted by the USAN council. American Medical Association.
- ↑ "Lilly, Incyte Treatment Shows Positive Results". www.insideindianabusiness.com. 9 Dec 2014. Retrieved 2 Mar 2015.