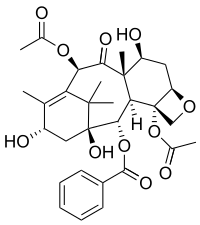
Baccatin III
150
 | |
| Names | |
|---|---|
| IUPAC name
(2β,5α,7α,10α,13β)-4,10-Diacetoxy-1,7,13-trihydroxy-9-oxo-5,20-epoxytax-11-en-2-yl benzoate | |
| Identifiers | |
| ChEBI | CHEBI:32898 |
| ChEMBL | ChEMBL288043 |
| ChemSpider | 23486966 |
| Jmol-3D images | Image |
| PubChem | 65366 |
| |
| Properties | |
| C31H38O11 | |
| Molar mass | 586.62677 Da |
| Melting point | 229 to 234 °C (444 to 453 °F; 502 to 507 K) |
| Acidity (pKa) | 12.76 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Baccatin III is an isolate of the Pacific yew tree (Taxus brevifolia) and related species. Baccatin III is a precursor to the anti-cancer drug paclitaxel (Taxol).
In 2014, researchers reported introduction and expression of the endophytic fungal gene responsible for synthesizing baccatin III (10-deacetylbaccatin III 10-O-acetyltransferase), to the mushroom Flammulina velutipes.[1] Researchers achieved the same acccoplishment with Escherichia coli in 2000.[2]
See also
References
- ↑ Han F, Kang LZ, Zeng XL, Ye ZW, Guo LQ, Lin JF (2014). "Bioproduction of baccatin III, an advanced precursor of paclitaxol with transgenic Flammulina velutipes expressing 10-Deacetylbaccatin III-10-O-acetyl transferase gene.". J Sci Food Agric 94: 2376–2383. doi:10.1002/jsfa.6562. PMID 24403190.
- ↑ Walker K, Croteau R (2000). "Molecular cloning of a 10-deacetylbaccatin III-10-O-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli.". Proc Natl Acad Sci U S A 97 (2): 583–7. doi:10.1073/pnas.97.2.583. PMC 15373. PMID 10639122.