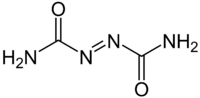
Azodicarbonamide
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Carbamoyliminourea | |
| Other names
Azodicarboxamide; Azobisformamide; C,C'-Azodi(formamide); Diazenedicarboxamide | |
| Identifiers | |
| 123-77-3 | |
| ChEMBL | ChEMBL28517 |
| ChemSpider | 4575589 |
| EC-number | 204-650-8 |
| |
| Jmol-3D images | Image |
| PubChem | 31269 |
| |
| UNII | 56Z28B9C8O |
| Properties | |
| Molecular formula |
C2H4N4O2 |
| Molar mass | 116.08 g·mol−1 |
| Appearance | Yellow to orange/red crystalline powder |
| Melting point | 225 °C (437 °F; 498 K) (decomposes) |
| Hazards | |
| MSDS | |
| EU classification | Harmful (XN) |
| R-phrases | R42 R44 |
| S-phrases | S22 S24 S37 |
| NFPA 704 | |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Azodicarbonamide, or azo(bis)formamide, is a chemical compound with the molecular formula C2H4O2N4.[1] It is a yellow to orange red, odorless, crystalline powder. As a food additive, it is known by the E number E927.
Uses
As a food additive
As a food additive, azodicarbonamide is used as a flour bleaching agent and a dough conditioner.[2] It reacts with moist flour as an oxidizing agent.[3] The main reaction product is biurea, a derivative of urea, which is stable during baking.[3] Secondary reaction products include semicarbazide and ethyl carbamate.[2]
Safety and regulation
As a food additive, azodicarbonamide is not authorised for use in Australia and the European Union.[4]:548[5] In the United States, azodicarbonamide has generally recognized as safe (GRAS) status and is allowed to be added to flour at levels up to 45 ppm.[6][4]:548
Other uses
The principal use of azodicarbonamide is in the production of foamed plastics as a blowing agent. The thermal decomposition of azodicarbonamide results in the evolution of nitrogen, carbon monoxide, carbon dioxide, and ammonia gases, which are trapped in the polymer as bubbles to form a foamed article.
Azodicarbonamide is used in plastics, synthetic leather, and other industries and can be pure or modified. Modification affects the reaction temperatures. Pure azodicarbonamide generally reacts around 200 °C. In the plastic, leather, and other industries, modified azodicarbonamide (average decomposition temperature 170 °C) contains additives that accelerate the reaction or react at lower temperatures.
Safety and regulation
Azodicarbonamide as a blowing agent in plastics has been banned in the European Union since August 2005 for the manufacture of plastic articles that are intended to come into direct contact with food.[7]
In the UK, the Health and Safety Executive has identified azodicarbonamide as a respiratory sensitizer (a possible cause of asthma) in workplace settings and determined that containers of it should be labeled with "May cause sensitisation by inhalation."[8] In a 1999 report, the World Health Organization linked azodicarbonamide to "respiratory issues, allergies and asthma" for individuals at workplaces where azodicarbonamide is manufactured or handled in raw form. The available data are restricted to these occupational environments. Exposure of the general public to azodicarbonamide could not be evaluated because of the lack of available data.[9] The WHO concluded, "The level of risk is uncertain; hence, exposure levels should be reduced as much as possible."[10]
References
- ↑ "Azodicarbonamide (CICADS)". Inchem. International Programme on Chemical Safety. Archived from the original on 24 August 2010. Retrieved 2010-08-14. Also published by World Health Organization, Geneva, 1999.
- ↑ 2.0 2.1 FDA Frequently Asked Questions on Azodicarbonamide (ADA) Page Last Updated: 20 June, 2014
- ↑ 3.0 3.1 WHO FAO 1965. Toxicological Evaluation of Some Antimicrobials, Antioxidants, Emulsifiers, Stabilizers, Flour-Treatment Agents, Acids and Bases: Azodicarbonamide FAO Nutrition Meetings Report Series No. 40A,B,C. WHO/Food Add./67.29
- ↑ 4.0 4.1 Smith, Jim; Hong-Shum, Lily (2011). Food additives data book (2nd ed.). Chichester, West Sussex: Wiley-Blackwell. ISBN 978-1405195430.
- ↑ European Commission. "European Union: Authorisation of Additives". Retrieved 2014-12-31.
- ↑ "21CFR172.806". Code of Federal Regulations. April 1, 2012.
- ↑ "COMMISSION DIRECTIVE 2004/1/EC of 6 January 2004 amending Directive 2002/72/EC as regards the suspension of the use of azodicarbonamide as blowing agent". Official Journal of the European Union. 2004-01-13. Retrieved 2011-03-10.
- ↑ "Substances causing/worsening asthma". UK Occupational Health and Safety. WorkSafe Victoria. Retrieved 2010-08-14.
- ↑ "Concise International Chemical Assessment Document 16: Azodicarbonamide" (PDF). World Health Organization. Retrieved 2014-02-05.
- ↑ Elizabeth Landau (17 February 2014). "Subway to remove 'dough conditioner' chemical from bread". CNN. Retrieved 23 January 2015.
External links
| ||||||
