Autocatalysis
A single chemical reaction is said to have undergone autocatalysis, or be autocatalytic, if the reaction product itself is the catalyst for that reaction.
A set of chemical reactions can be said to be "collectively autocatalytic" if a number of those reactions produce, as reaction products, catalysts for enough of the other reactions that the entire set of chemical reactions is self-sustaining given an input of energy and food molecules (see autocatalytic set).
Rate law in autocatalytic reactions
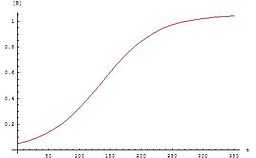
The rate law for the second order autocatalytic reaction
is the following one
![\ v = k[A][B]](../I/m/de10acc5c8a835e03ff527ffd5d6a61c.png) .
.
The concentrations of A and B vary in time according to
and
![[B]=\frac{[A]_0+[B]_0}{1+\frac{[A]_0}{[B]_0}e^{-([A]_0+[B]_0)kt}}](../I/m/a2e8f292ed1676b68823e5e5bdcff432.png) .
.
The graph for these equations is a sigmoid curve, which is typical for autocatalytic reactions: these chemical reactions proceed slowly at the start because there is little catalyst present, the rate of reaction increases progressively as the reaction proceeds as the amount of catalyst increases and then it again slows down as the reactant concentration decreases. If the concentration of a reactant or product in an experiment follows a sigmoid curve, the reaction is likely to be autocatalytic.
Asymmetric autocatalysis
Asymmetric autocatalysis occurs when the reaction product is chiral and thus acts as a chiral catalyst for its own production. Reactions of this type, such as the Soai reaction, have the property that they can amplify a very small enantiomeric excess into a large one. This has been proposed as an important step in the origin of biological homochirality.[1]
Role in origin of life
In 1995 Stuart Kauffman proposed that life initially arose as autocatalytic chemical networks.[2]
British ethologist Richard Dawkins wrote about autocatalysis as a potential explanation for abiogenesis in his 2004 book The Ancestor's Tale. He cites experiments performed by Julius Rebek and his colleagues at the Scripps Research Institute in California in which they combined amino adenosine and pentafluorophenyl ester with the autocatalyst amino adenosine triacid ester (AATE). One system from the experiment contained variants of AATE which catalysed the synthesis of themselves. This experiment demonstrated the possibility that autocatalysts could exhibit competition within a population of entities with heredity, which could be interpreted as a rudimentary form of natural selection, and that certain environmental changes (such as irradiation) could alter the chemical structure of some of these self-replicating molecules (an analogue for mutation) in such ways that could either boost or interfere with its ability to react, thus boosting or interfering with its ability to replicate and spread in the population.[3]
Autocatalysis plays a major role in the processes of life. Two researchers who have emphasised its role in the origins of life are Robert Ulanowicz [4] and Stuart Kauffman.[5]
Autocatalysis occurs in the initial transcripts of rRNA. The introns are capable of excising themselves by the process of two nucleophilic transesterification reactions. The RNA able to do this is sometimes referred to as a ribozyme. Additionally, the citric acid cycle is an autocatalytic cycle run in reverse.
Ultimately, biological metabolism itself can be seen as a vast autocatalytic set, in that all of the molecular constituents of a biological cell are produced by reactions involving this same set of molecules.
Examples of autocatalytic reactions
- DNA replication
- Haloform reaction
- Tin pest
- Reaction of Permanganate with Oxalic Acid
- The mechanism of the above reaction [6]
- Vinegar syndrome
- Binding of oxygen by hemoglobin
- The spontaneous degradation of aspirin into salicylic acid and acetic acid, causing very old aspirin in sealed containers to smell mildly of vinegar.
- The α-bromination of acetophenone with bromine.
See also
- Autocatalytic reactions and order creation
References
- ↑ Soai K, Sato I, Shibata T (2001). "Asymmetric autocatalysis and the origin of chiral homogeneity in organic compounds.". Chem Rec 1 (4): 321–32. doi:10.1002/tcr.1017. PMID 11893072.
- ↑ Stuart Kauffman (1995). At Home in the Universe: The Search for the Laws of Self-Organization and Complexity. Oxford University Press. ISBN 0-19-509599-5.
- ↑ Rebeck, Julius (July 1994). "Synthetic Self-Replicating Molecules". Scientific American: 48–55.
- ↑ Ecology, the Ascendent Perspective", Robert Ulanowicz, Columbia Univ. Press 1997
- ↑ Investigations, Stuart Kauffman.
- ↑ Kovacs KA, Grof P, Burai L, Riedel M (2004). "Revising the Mechanism of the Permanganate/Oxalate Reaction". J. Phys. Chem. A 108 (50): 11026. doi:10.1021/jp047061u.
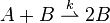
![[A]=\frac{[A]_0+[B]_0}{1+\frac{[B]_0}{[A]_0}e^{([A]_0+[B]_0)kt}}](../I/m/702f04dd65537d65853d6785c376928d.png)