Ataluren
 | |
| Names | |
|---|---|
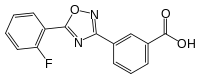
| IUPAC name
3-[5-(2-Fluorophenyl)-1,2,4-oxadiazol-3-yl]benzoic acid | |
| Other names
PTC124 | |
| Identifiers | |
| 775304-57-9 | |
| ChEMBL | ChEMBL256997 |
| ChemSpider | 9394889 |
| |
| Jmol-3D images | Image |
| KEGG | D09323 |
| PubChem | 11219835 |
| |
| Properties | |
| C15H9FN2O3 | |
| Molar mass | 284.24 g/mol |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Ataluren, formerly known as PTC124, is a small-molecular agent designed by PTC Therapeutics and sold under the trade name Translarna in the European Union.
Mechanism
Ataluren makes ribosomes less sensitive to premature stop codons (referred to as "read-through"). This may be beneficial in diseases such as Duchenne muscular dystrophy where the mRNA contains a mutation causing premature stop codons or nonsense codons. There is ongoing debate over whether Ataluren is truly a functional drug (inducing codon read-through), or if it is nonfunctional, and the result was a false-positive hit from a biochemical screen based on luciferase.[1]
In cystic fibrosis, early studies of ataluren show that it improves nasal potential difference.[2] Ataluren appears to be most effective for the stop codon 'UGA'.[3]
Clinical
Ataluren has been tested on healthy humans and humans carrying genetic disorders caused by nonsense mutations,[3][4] such as some people with cystic fibrosis and Duchenne muscular dystrophy.
In 2010, PTC Therapeutics released preliminary results of its phase 2b clinical trial for Duchenne muscular dystrophy, with participants not showing a significant improvement in the six minute walk distance after the 48 weeks of the trial.[5] This failure resulted in the termination of a $100 million deal with Genzyme to pursue the drug.
Phase 2 clinical trials were successful for cystic fibrosis in Israel, France and Belgium.[6] Multicountry phase 3 clinical trials are currently in progress for cystic fibrosis in Europe and the USA.[7]
Approval
On 23 May 2014 ataluren received a positive opinion from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA).[8] Translarna was first available in Germany, the first EU country to launch the new medicine.[9]
In August 2014, ataluren received market authorization from the European Commission to treat patients with nonsense mutation Duchenne muscular dystrophy. A confirmatory phase III clinical trial is ongoing.[9] The drug does not yet have approval by the US Food and Drug Administration.
See also
- Biostrophin, another drug against Duchenne muscular dystrophy
- Ivacaftor and lumacaftor, other drugs against cystic fibrosis in development by Vertex Pharmaceuticals
References
- ↑ Derek (2013-09-18). "The Arguing Over PTC124 and Duchenne Muscular Dystrophy. In the Pipeline:". Pipeline.corante.com. Retrieved 2013-11-28.
- ↑ Wilschanski, M. (2013). "Novel therapeutic approaches for cystic fibrosis". Discovery medicine 15 (81): 127–133. PMID 23449115.
- ↑ 3.0 3.1 Welch EM, Barton ER, Zhuo J, Tomizawa Y, Friesen WJ, Trifillis P, Paushkin S, Patel M, Trotta CR, Hwang S, Wilde RG, Karp G, Takasugi J, Chen G, Jones S, Ren H, Moon YC, Corson D, Turpoff AA, Campbell JA, Conn MM, Khan A, Almstead NG, Hedrick J, Mollin A, Risher N, Weetall M, Yeh S, Branstrom AA, Colacino JM, Babiak J, Ju WD, Hirawat S, Northcutt VJ, Miller LL, Spatrick P, He F, Kawana M, Feng H, Jacobson A, Peltz SW, Sweeney HL (May 2007). "PTC124 targets genetic disorders caused by nonsense mutations". Nature 447 (7140): 87–91. doi:10.1038/nature05756. PMID 17450125.
- ↑ Hirawat S, Welch EM, Elfring GL, Northcutt VJ, Paushkin S, Hwang S, Leonard EM, Almstead NG, Ju W, Peltz SW, Miller LL (Apr 2007). "Safety, tolerability, and pharmacokinetics of PTC124, a nonaminoglycoside nonsense mutation suppressor, following single- and multiple-dose administration to healthy male and female adult volunteers". Journal of clinical pharmacology 47 (4): 430–444. doi:10.1177/0091270006297140. PMID 17389552.
- ↑ "PTC THERAPEUTICS AND GENZYME CORPORATION ANNOUNCE PRELIMINARY RESULTS FROM THE PHASE 2B CLINICAL TRIAL OF ATALUREN FOR NONSENSE MUTATION DUCHENNE/BECKER MUSCULAR DYSTROPHY (NASDAQ:PTCT)". Ptct.client.shareholder.com. Retrieved 2013-11-28.
- ↑ Wilschanski, M.; Miller, L. L.; Shoseyov, D.; Blau, H.; Rivlin, J.; Aviram, M.; Cohen, M.; Armoni, S.; Yaakov, Y.; Pugatsch, T.; Cohen-Cymberknoh, M.; Miller, N. L.; Reha, A.; Northcutt, V. J.; Hirawat, S.; Donnelly, K.; Elfring, G. L.; Ajayi, T.; Kerem, E. (2011). "Chronic ataluren (PTC124) treatment of nonsense mutation cystic fibrosis". European Respiratory Journal 38 (1): 59–69. doi:10.1183/09031936.00120910. PMID 21233271. Sermet-Gaudelus, I.; Boeck, K. D.; Casimir, G. J.; Vermeulen, F.; Leal, T.; Mogenet, A.; Roussel, D.; Fritsch, J.; Hanssens, L.; Hirawat, S.; Miller, N. L.; Constantine, S.; Reha, A.; Ajayi, T.; Elfring, G. L.; Miller, L. L. (November 2010). "Ataluren (PTC124) induces cystic fibrosis transmembrane conductance regulator protein expression and activity in children with nonsense mutation cystic fibrosis". American Journal of Respiratory and Critical Care Medicine 182 (10): 1262–1272. doi:10.1164/rccm.201001-0137OC. PMID 20622033.
- ↑ "PTC Therapeutics Completes Enrollment of Phase 3 Trial of Ataluren in Patients with Cystic Fibrosis (NASDAQ:PTCT)". Ptct.client.shareholder.com. 2010-12-21. Retrieved 2013-11-28.
- ↑ http://www.marketwatch.com/story/ptc-therapeutics-receives-positive-opinion-from-chmp-for-translarna-ataluren-2014-05-23
- ↑ 9.0 9.1 "PTC Therapeutics Announces Launch of Translarna™ (ataluren) in Germany". marketwatch.com. 3 Dec 2014. Retrieved 27 Dec 2014.