Artemether
 | |
| Systematic (IUPAC) name | |
|---|---|
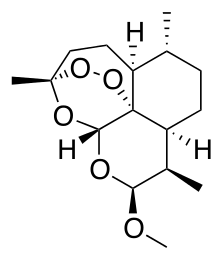
| (3R,5aS,6R,8aS,9R,10S,12R,12aR)-10-methoxy-3,6,9-trimethyldecahydro-12H-3,12-epoxy[1,2]dioxepino[4,3-i]-2-benzopyran | |
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| |
| |
| Oral | |
| Identifiers | |
|
71963-77-4 | |
| P01BE02 | |
| PubChem | CID 68911 |
| DrugBank |
DB06697 |
| ChemSpider |
62138 |
| UNII |
C7D6T3H22J |
| KEGG |
D02483 |
| ChEBI |
CHEBI:195280 |
| ChEMBL |
CHEMBL1237051 |
| Chemical data | |
| Formula | C16H26O5 |
| 298.374 g/mol | |
|
SMILES
| |
| |
| | |
Artemether (INN) is an antimalarial for the treatment of multiple drug-resistant strains of Plasmodium falciparum malaria. Its combination (coformulation) with Lumefantrine has first been marketed by Novartis under the brand names Riamet and Coartem. Today, this combination therapy is available as generic from several manufacturers.
It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[1]
Medical uses
Antimalarial
Artemether is effective against the blood schizonts of both malarial parasites P. falciparum and P. vivax. It, however, may not be as good as artesunate for severe malaria.[2]
It is available as monotherapy, but usually applied in combination with lumefantrine in treatments of malaria. World Health Organization guidelines for the treatment of uncomplicated falciparum malaria recommend the use of this artemisinin-based combination therapy. Zambia was the first African country to adopt artemether/lumefantrine (commonly called Coartem) as first-line therapy in national malaria treatment guidelines in 2002. Clinical records show that by 2008, the rates of in-patient malaria cases and deaths decreased by 61% and 66%, respectively, compared with the 2001-2002 reference period. In South Africa, also, the number of malaria-related outpatient cases and hospital admissions of each fell by 99% from 2001 to 2003, and malaria-related deaths decreased by 97% over the same period.[3]
The efficacy of the six-dose regimen of Coartem has been confirmed in many different populations around the world, consistently achieving 28-day polymerase chain reaction-corrected cure rates of >95% in the evaluable population, rapidly clearing parasitaemia and fever, and demonstrating a significant gametocidal effect, even in areas of widespread parasite resistance to other antimalarials.[4] Coartem is more effective than quinine, the classical antimalarial. For P. vivax infection, combination with piperaquine is more effective than Coartem.[5]
Artemether has been assigned to category C by the FDA on the basis of animal data which show an association with fetal loss and deformity. However, clinical data appear to show that artemether is safe in pregnancy. A meta-analysis that looked at artemether in 945 pregnant women did not find evidence of harm,[6] and a clinical trial of artemether-lumefantrine designed to look at this question found fewer adverse events in the 138 pregnant women treated with artemther-lumefantrine than women treated with quinine.[7]
Anthelmintic
During the early 1980s, Chinese scientists, by serendipity, discovered that artemether was not only an antimalarial agent, but also effective against the blood flukes. Eventually, laboratory experiments have confirmed the broad spectrum of activity against different trematodes, including all human schistosomes, Clonorchis sinensis, Fasciola hepatica, and Opisthorchis viverrini.[8] These studies revealed that artemether exhibits the highest activity against juvenile stages of the trematodes, while adult worms are significantly less susceptible. In addition, no indication of neurotoxicity was seen following repeated high doses. Randomized controlled clinical trials confirmed that artemether, orally administered at a dose of 6 mg/kg once every 2–3 weeks, results in no drug-related adverse effects, and significantly reduces the incidence and intensity of schistosome infections, including those of Schistosoma mansoni, S. japonicum, and S. haematobium.[9][10]
Chemical nature
It is a methyl ether derivative of artemisinin, which is a peroxide lactone isolated from the antimalarial plant Artemisia annua. It is also known as dihydroartemisinin methyl ether, but its correct chemical nomenclature is (+)-(3-alpha,5a-beta,6-beta,8a-beta, 9-alpha,12-beta,12aR)-decahydro-10-methoxy-3,6,9-trimethyl-3,12-epoxy-12H-pyrano(4,3-j)-1,2-benzodioxepin. It is a relatively lipophilic and unstable drug.[11]
Pharmacokinetics and pharmacodynamics
Artemether interacts with ferriprotoporphyrin IX (“heme”), or ferrous ions, in the acidic parasite food vacuole, which results in the generation of cytotoxic radical species. The generally accepted mechanism of action of peroxide antimalarials involves interaction of the peroxide-containing drug with heme, a hemoglobin degradation byproduct, derived from proteolysis of hemoglobin. This interaction is believed to result in the formation of a range of potentially toxic oxygen and carbon-centered radicals. Numerous studies have investigated the type of damage oxygen radicals may induce. For example, Pandey et al. have observed inhibition of digestive vacuole cysteine protease activity of malarial parasites by artemether. These observations were supported by ex vivo experiments showing accumulation of hemoglobin in the parasites treated with artemether and inhibition of hemozoin formation by malaria parasites. Electron microscopic evidence linking artemisinin action to the parasite's digestive vacuole has been obtained showing that the digestive vacuole membrane suffers damage soon after parasites are exposed to artemether. This would also be consistent with data showing that the digestive vacuole is already established by the mid-ring stage of the parasite's blood cycle, a stage that is sensitive to artemether, but not other antimalarials. A commonly cited theory that the parasite's SERCA pump (PfATP6 / PfSERCA) is a target of artemether has been increasingly questioned by some, although this hypothesis has been discussed in detail by others . It is now clear that the original studies claiming specific interactions between SERCAs and artemether were undertaken in a Xenopus oocyte system with a poor signal:noise ratio. Other mechanisms of action for artemether include their ability to reduce fever by production of signals to hypothalamus thermoregulatory center. Now, recent research has shown the presence of a new, previously unknown cyclooxygenase enzyme COX-3, found in the brain and spinal cord, which is selectively inhibited by artemether, and is distinct from the two already known cyclooxygenase enzymes COX-1 and COX-2. It is now believed that this selective inhibition of the enzyme COX-3 in the brain and spinal cord explains the ability of artemether in relieving pain and reducing fever which is produced by malaria.
References
- ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ Esu, E; Effa, EE; Opie, ON; Uwaoma, A; Meremikwu, MM (Sep 11, 2014). "Artemether for severe malaria.". The Cochrane database of systematic reviews 9: CD010678. doi:10.1002/14651858.CD010678.pub2. PMID 25209020.
- ↑ Barnes KI, Chanda P, Ab Barnabas G (2009). "Impact of the large-scale deployment of artemether/lumefantrine on the malaria disease burden in Africa: case studies of South Africa, Zambia and Ethiopia". Malar J. 8 (1): S8. doi:10.1186/1475-2875-8-S1-S8. PMC 2760243. PMID 19818175.
- ↑ Makanga M, Krudsood S (2009). "The clinical efficacy of artemether/lumefantrine (Coartem®)". Malar J. 8 (1): S5. doi:10.1186/1475-2875-8-S1-S5. PMC 2760240. PMID 19818172.
- ↑ Sinclair D, Zani B, Donegan S, Olliaro P, Garner P (2009). Sinclair, David, ed. "Artemisinin-based combination therapy for treating uncomplicated malaria". Cochrane Database Syst Rev. 8 (3): CD007483. doi:10.1002/14651858.CD007483.pub2. PMID 19588433.
- ↑ Dellicour S, Hall S, Chandramohan D, Greenwood B (2007). "The safety of artemisinins during pregnancy: a pressing question". Malaria J 6: 15. doi:10.1186/1475-2875-6-15.
- ↑ Piola P, Nabasumba C, Turyakira E et al. (2010). "Efficacy and safety of artemether—lumefantrine compared with quinine in pregnant women with uncomplicated Plasmodium falciparum malaria: an open-label, randomised, non-inferiority trial". Lancet Infect Dis 10 (11): 762–769. doi:10.1016/S1473-3099(10)70202-4.
- ↑ Keiser J, Utzinger J (2007). "Artemisinins and synthetic trioxolanes in the treatment of helminth infections". Curr Opin Infect Dis 20 (6): 605–612. doi:10.1097/QCO.0b013e3282f19ec4. PMID 17975411.
- ↑ Xiao S, Tanner M, N'Goran EK, Utzinger J, Chollet J, Bergquist R, Chen M, Zheng J (2002). "Recent investigations of artemether, a novel agent for the prevention of schistosomiasis japonica, mansoni and haematobia". Acta Trop 82 (2): 175–181. doi:10.1016/S0001-706X(02)00009-8. PMID 12020890.
- ↑ Hou XY, McManus DP, Gray DJ, Balen J, Luo XS, He YK, Ellis M, Williams GM, Li YS (2008). "A randomized, double-blind, placebo-controlled trial of safety and efficacy of combined praziquantel and artemether treatment for acute schistosomiasis japonica in China". Bull World Health Organ 86 (10): 788–795. doi:10.2471/BLT.08.053041. PMC 2649525. PMID 18949216.
- ↑ B.M.J. De Spiegeleer, M. D’Hondt, E. Vangheluwe, K. Vandercruyssen, B.G.I. De Spiegeleer, H. Jansen, I. Koijen, J. Van Gompel. Relative response factor determination of artemether degradants with a dry heat stress approach. Journal of Pharmaceutical and Biomedical Analysis 70 (2012) 111– 116.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||