Arsenic pentoxide
 | |
| Names | |
|---|---|
| Other names
Arsenic(V) oxide Arsenic oxide Arsenic anhydride | |
| Identifiers | |
| 1303-28-2 | |
| ChemSpider | 14088 |
| EC number | 215-116-9 |
| |
| Jmol-3D images | Image |
| PubChem | 14771 |
| RTECS number | CG2275000 |
| |
| Properties | |
| As2O5 | |
| Molar mass | 229.8402 g/mol |
| Appearance | white hygroscopic powder |
| Density | 4.32 g/cm3 |
| Melting point | 315 °C (599 °F; 588 K) (decomposes) |
| 59.5 g/100 mL (0 °C) 65.8 g/100 mL (20 °C) 8.20 g/100 mL (100 °C) | |
| Solubility | soluble in alcohol |
| Acidity (pKa) | 7 |
| Hazards | |
| EU classification | Very toxic (T+) Carc. Cat. 1 Dangerous for the environment (N) |
| R-phrases | R45, R23/25, R50/53 |
| S-phrases | S53, S45, S60, S61 |
| NFPA 704 | |
| LD50 (Median lethal dose) |
8 mg/kg (rat, oral) |
| Related compounds | |
| Other cations |
Phosphorus pentoxide Antimony pentoxide |
| Related compounds |
Arsenic trioxide Arsenic acid |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Arsenic pentoxide is the inorganic compound with the formula As2O5.[1] This glassy, white, deliquescent solid is relatively unstable, consistent with the rarity of the As(V) oxidation state. More common, and far more important commercially, is arsenic(III) oxide (As2O3). All arsenic compounds are highly toxic and thus find only limited commercial applications.
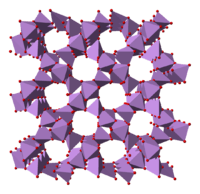
Structure
The structure consists of tetrahedral {AsO4} and octahedral {AsO6} centres linked by sharing corners.[2] The structure differs from that of the corresponding phosphorus(V) oxide; as a result, although there is still a solid solution with that oxide, it only progresses to the equimolar point, at which point phosphorus has substituted for arsenic in all of its tetrahedral sites. Likewise, arsenic pentoxide can also dissolve up to an equimolar amount of antimony pentoxide, as antimony substitutes for arsenic only in its octahedral sites.[3]
Synthesis
Historical
Paracelsus Macquer found a crystallizable salt which he called ‘sel neutre arsenical’. This salt was the obtaining residue after distilling nitric acid from a mixture of potassium nitrate and arsenic trioxide. Previously Paracelsus heated a mixture of arsenic trioxide and potassium nitrate. He applied the term ‘arsenicum fixum’ to the product. A. Libavius called the same product ‘butyrum arsenici’ (butter of arsenic), although this term was actually used for arsenic tricholoride. The products that Paracelsus and Libavius found were all impure alkali arsenates.[4] Scheele prepared a number of arsenates by the action of arsenic acid on the alkalies. One of the arsenates that he prepared, was arsenic pentoxide.[5] The water in the alkalies evaporated at 180˚C, and the arsenic pentoxide was stable below 400˚C .[4]
Modern methods
Arsenic pentoxide can be crystallized by heating As2O3 under oxygen. This reaction is reversible:[2]
As2O5  As2O3 + O2
As2O3 + O2
Strong oxidizing agents such as ozone, hydrogen peroxide, and nitric acid convert arsenic trioxide to the pentoxide.
Arsenic acid can be generated via routine processing of arsenic compounds including the oxidation of arsenic and arsenic-containing minerals in air. Illustrative is the roasting of orpiment, a typical arsenic sulfide ore:[6] 2 As2S3 + 11 O2 → 2 As2O5 + 6 SO2
Safety
Like all arsenic compounds, the pentoxide is highly toxic. Its reduced derivative arsenite, which is an As(III) compound, is even more toxic since it has a high affinity for thiol groups of cysteine residues in proteins.
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0080379419.
- ↑ 2.0 2.1 Martin Jansen (1977). "Crystal Structure of As2O5". Angewandte Chemie International Edition in English 16 (5): 314–315. doi:10.1002/anie.197703142.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0080379419.
- ↑ 4.0 4.1 J. W. Mellor. "Comprehensive Treatise on Inorganic & Theoretical Chemistry". http://www.rexresearch.com/alchemy10/melloras.htm English. IX - Arsenic.
- ↑ C.W. Zenger et al. "Arsenic 149". http://tera-3.ul.cs.cmu.edu/NASD/4dcb85c3-9fee-4c83-9e6d-fe6ce5522b59/China/disk4/75/75-2/31005153/HTML/00000166.htm''.
- ↑ Grund, S. C.; Hanusch, K.; Wolf, H. U. (2005), "Arsenic and Arsenic Compounds", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a03_113.pub2
External links
- NIOSH Pocket Guide to Chemical Hazards
- IARC Monograph – Arsenic and Arsenic Compounds
- NTP Report on Carcinogens – Inorganic Arsenic Compounds
- ESIS: European chemical Substances Information System
- Linstrom, P.J.; Mallard, W.G. (eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69. National Institute of Standards and Technology, Gaithersburg MD. http://webbook.nist.gov
| ||||||
