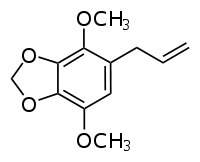
Apiol
 | |
 | |
| Names | |
|---|---|
| IUPAC names
1-allyl-2,5-dimethoxy- 3,4-methylenedioxybenzene | |
| Identifiers | |
| 523-80-8 | |
| ChEMBL | ChEMBL984002 |
| ChemSpider | 21106259 |
| |
| Jmol-3D images | Image |
| KEGG | C10429 |
| |
| UNII | QQ67504PXO |
| Properties | |
| C12H14O4 | |
| Molar mass | 222.23 g/mol |
| Density | 1.151 g/mL |
| Melting point | 30 °C (86 °F; 303 K) |
| Boiling point | 294 °C (561 °F; 567 K) |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Apiol is a phthalide, also known as parsley apiol, apiole or parsley camphor. It is found in the essential oils of celery leaf and all parts of parsley.[1] Heinrich Christoph Link, an apothecary in Leipzig, discovered the substance in 1715 as greenish crystals reduced by steam from oil of parsley.[2] In 1855 Joret and Homolle discovered that apiol was an effective treatment of amenorrea or lack of menstruation.
In medicine it has been used, as essential oil or in purified form, for the treatment of menstrual disorders and as an abortifacient. It is an irritant and, in high doses, it can cause liver and kidney damage.[3] Cases of death due to attempted abortion using apiol have been reported.[4][5]
Hippocrates wrote about parsley as a herb to cause an abortion.[6] Plants containing apiol were used by women in the Middle Ages to terminate pregnancies. Its use was widespread in the USA, often as ergoapiol or apergol, until a highly toxic adulterated product containing apiol and tri-ortho-cresyl phosphate (also famous as the adulterant added to Jamaican ginger) was introduced on the American market. No carcinogenicity was detected with parsley apiol or dill apiol in mice.[7]
Now that safer methods of abortion are available, apiol is almost forgotten.
The name apiol is also used for other closely related compounds, found in dill (dillapiole, 1-allyl-2,3-dimethoxy-4,5-methylenedioxybenzene) and in fennel roots.
See also
References
- ↑ Azeez, Shamina; Krishnamurthy, K. (2008). Chemistry of Spices. Calicut, Kerala, India: Biddles Ltd. pp. 380 & 404. ISBN 9781845934057.
- ↑ Shorter, Edward (1991). Women's Bodies: A Social History of Women's Encounter With Health, Ill-Health, and Medicine. New Brunswick, NJ: Transaction Publishers.
- ↑ Amerio A; De Benedictis G; Leondeff J et al. (Jan–Apr 1968). "Nephropathy due to apiol". Minerva Nefrol (in Italian) 15 (1). pp. 49–70.
- ↑ Quinn LJ; Harris C; Joron GE (Apr 15, 1958). "Apiol poisoning". Can Med Assoc J 78 (8). pp. 635–6.
- ↑ Hermann K; Le Roux A; Fiddes FS (Jun 16, 1956). "Death from apiol used as abortifacient". Lancet 270 (6929). pp. 937–9.
- ↑ Sage-Femme Collective (2008). Natural Liberty: Rediscovering Self-Induced Abortion Methods. Sage-Femme Collective. ISBN 978-0964592001.
- ↑ Phillips DH; Reddy MV; Randerath K (1984). "32P-post-labelling analysis of DNA adducts formed in the livers of animals treated with safrole, estragole and other naturally-occurring alkenylbenzenes. II. Newborn male B6C3F1 mice". Carcinogenesis 5 (12). pp. 1623–8.
External links
- Apiol chemical information from chemindustry.com
- Apiol in the ChemIDplus database
- Essential oil from fennel plants--studies on the composition