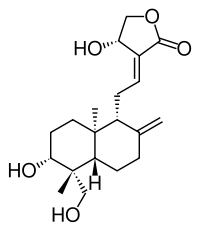
Andrographolide
 | |
| Names | |
|---|---|
| IUPAC name
3-[2-[decahydro-6-hydroxy-5- (hydroxymethyl)-5,8a- dimethyl-2-methylene-1- napthalenyl]ethylidene]dihydro- 4-hydroxy-2(3H)-furanone | |
| Identifiers | |
| 5508-58-7 | |
| ChEBI | CHEBI:65408 |
| ChEMBL | ChEMBL186141 |
| ChemSpider | 16735664 |
| |
| Jmol-3D images | Image Image |
| PubChem | 5318517 |
| |
| UNII | 410105JHGR |
| Properties | |
| C20H30O5 | |
| Molar mass | 350.45 g/mol |
| Appearance | Rhombic prisms or plates from ethanol or methanol |
| Density | 1.2317 g/cm3 |
| Melting point | 230 to 231 °C (446 to 448 °F; 503 to 504 K) |
| Sparingly soluble | |
| Related compounds | |
| Related labdanes |
14-deoxyandrographolide |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Andrographolide is a labdane diterpenoid that is the main bioactive component of the medicinal plant Andrographis paniculata.[1] Andrographolide is an extremely bitter substance extracted from the stem and leaves of A. paniculata.
Andrographolide is used experimentally in different areas of research including cell signaling, immunomodulation, and stroke.[2]
Mechanism of Action - Drug target - Andrographolide targets
Study has shown that Andrographlide may bind to a spectrum of protein targets by covalent modification in cancer cells, including NF-κB, actin, etc.[3]
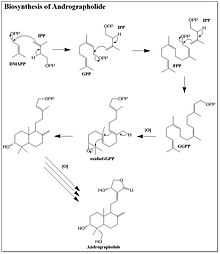
Biosynthesis
While Andrographolide is a relatively simple diterpene lactone, the biosynthesis by Andrographis paniculata was only recently determined. Andrographolide is a member of the isoprenoid family of natural products. The precursors to isoprenoid biosynthesis, isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), can be synthesized through either the Mevalonic Acid (MVA) or deoxyxylulose (DXP) pathway.[4] Through selective C13 labeling of the precursors to both the MVA and DXP pathways, it was determined that the majority of the Andrographolide precursors are synthesized through the DXP pathway.[5] It is worth noting that there is still a small portion of Andrographolide precursors are synthesized through the MVA pathway. The biosynthesis of Andrographolide begins with the addition of IPP to DMAPP, which forms GPP. Another molecule of IPP is then added, yielding FPP. The final IPP molecule is added to the FPP to complete the backbone of the diterpene. The double bond originating from DMAPP is oxidized to an epoxide prior to the ring closing cascade that forms two six-membered rings. A series of oxidations form a five-membered lactone in addition to adding on the alcohol groups. The order of these post-synthetic modifications is not entirely known.[6]
References
- ↑ Chakravarti RN, Chakravarti D (1951). "Andrographolide, the active constituent of Andrographis paniculata Nees; a preliminary communication". Ind Med Gaz 86 (3): 96–7. PMID 14860885.
- ↑ abcamBiochemicals Andrographolide-ab120636
- ↑ Wang, J; Tan, X. F.; Nguyen, V. S.; Yang, P; Zhou, J; Gao, M; Li, Z; Lim, T. K.; He, Y; Ong, C. S.; Lay, Y; Zhang, J; Zhu, G; Lai, S. L.; Ghosh, D; Mok, Y. K.; Shen, H. M.; Lin, Q (2014). "A quantitative chemical proteomics approach to profile the specific cellular targets of andrographolide, a promising anticancer agent that suppresses tumor metastasis". Molecular & Cellular Proteomics 13 (3): 876–86. doi:10.1074/mcp.M113.029793. PMC 3945915. PMID 24445406.
- ↑ Srivastava, N; Akhila, A (2010). "Biosynthesis of andrographolide in Andrographis paniculata". Phytochemistry 71 (11–12): 1298–304. doi:10.1016/j.phytochem.2010.05.022. PMID 20557910.
- ↑ Srivastava, N; Akhila, A (2010). "Biosynthesis of andrographolide in Andrographis paniculata". Phytochemistry 71 (11–12): 1298–304. doi:10.1016/j.phytochem.2010.05.022. PMID 20557910.
- ↑ Srivastava, N; Akhila, A (2010). "Biosynthesis of andrographolide in Andrographis paniculata". Phytochemistry 71 (11–12): 1298–304. doi:10.1016/j.phytochem.2010.05.022. PMID 20557910.