Amurensin A
Amurensin A
 |
| Identifiers |
| Jmol-3D images |
Image |
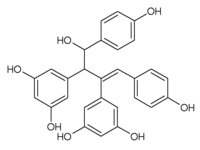
Oc1cc(cc(O)c1)C(\C(=C\c2ccc(O)cc2)c3cc(O)cc(O)c3)C(O)c4ccc(O)cc4
|
| Properties |
| |
C28H24O7 |
| Molar mass |
472.48 g/mol |
Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) |
|
| Infobox references |
|
|
Amurensin A is an oligostilbene found in Vitis amurensis.[1] It is a resveratrol dimer with a C8-C8' connection.[2]
References
- ↑ Huang, K. S.; Lin, M. (1999). "Oligostilbenes from the Roots of Vitis amurensis". Journal of Asian Natural Products Research 2 (1): 21–28. doi:10.1080/10286029908039886. PMID 11261202.
- ↑ Natural stilbenes: an overview. Tao Shen , Xiao-Ning Wang and Hong-Xiang Lou, Nat. Prod. Rep.,2009, 26, pages 916-935, doi:10.1039/B905960A
|
|---|
|
- Diptoindonesin C
- Diptoindonesin F
- Gnetin H
- Hemsleyanol D
- Isohopeaphenol
- Laetevirenol A, B, C, D and E
- Suffruticosol A and B
- Viniferal
- E-ω-viniferin
- Z-ω-viniferin
| | | Dimers |
- Diptoindonesin G
- Jezonodione
- B
- Scirpusin A
- Tibeticanol (piceatannol dimer)
|
|---|
| | Trimers |
- Amurensin B
- Gnetin E
- Gneyulin A
- Johorenol A
- Ampelopsin E
- Vaticanol G
|
|---|
| | Tetramers: |
- Dibalanocarpol
- Gnetin J (3"-hydroxygnetin E)
- Gnetin K (3"-methoxygnetin E)
- Gnetuhainin R (isorhapontigenin tetramer)
- Laetevirenol F and G
|
|---|
| Higher polymers
(five units or more) | |
|---|
| Oligomeric forms
of resveratrol | Dimers | |
|---|
| Trimers | |
|---|
| Tetramers | |
|---|
| Pentamers | |
|---|
| Hexamers | |
|---|
| Higher polymers |
- γ-viniferin
- Valeriaphenol A
|
|---|
|
|---|
| | Glycosides or conjugates |
- Diptoindonesin A (C-glucoside of ε-viniferin)
- Foeniculoside I (glucoside of miyabenol C), II, III and IV
- Laevifonol (an ε-viniferin-ascorbic acid hybrid compound)
- Laevifoside (O-glucoside of ampelopsin A)
|
|---|
|
