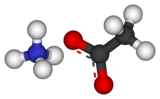
Ammonium acetate
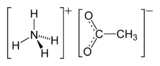 | |
 | |
| | |
| Names | |
|---|---|
| IUPAC name
Ammonium ethanoate | |
| Identifiers | |
| 631-61-8 | |
| ChEBI | CHEBI:62947 |
| ChemSpider | 11925 |
| |
| Jmol-3D images | Image |
| PubChem | 517165 |
| RTECS number | AF3675000 |
| |
| UNII | RRE756S6Q2 |
| Properties | |
| Molecular formula |
C2H7NO2 |
| Molar mass | 77.08 g·mol−1 |
| Appearance | White solid crystals, deliquescent |
| Odor | Slighty acetic |
| Density | 1.17 g/cm3 (20 °C)[1] 1.073 g/cm3 (25 °C) |
| Melting point | 110–114 °C (230–237 °F; 383–387 K) [2][1] decomposes[3] |
| Boiling point | 117.1 °C (242.8 °F; 390.2 K) at 760 mmHg, decomposes |
| 102 g/100 mL (0 °C) 148 g/100 mL (4 °C)[1] 143 g/100 mL (20 °C) 533 g/100 mL (80 °C) | |
| Solubility | Soluble in alcohol, SO2, acetone, liquid ammonia[3] |
| Solubility in methanol | 7.89 g/100 mL (15 °C)[2][1] 131.24 g/100 g (94.2 °C)[3] |
| Solubility in dimethylformamide | 0.1 g/100 g[3] |
| Structure | |
| Crystal structure | Orthorhombic |
| Thermochemistry | |
| Std enthalpy of formation (ΔfH |
−615 kJ/mol[3] |
| Hazards | |
| MSDS | JT Baker |
| Main hazards | Irritant |
| GHS pictograms |  [2] [2] |
| GHS signal word | Warning |
| H303, H316, H320, H333[2] | |
| P281, P335[2] | |
| NFPA 704 | |
| Flash point | 136 °C (277 °F; 409 K) [2] |
| LD50 (Median lethal dose) |
386 mg/kg (mice, intravenous)[3] |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Ammonium acetate is a chemical compound with the formula NH4C2H3O2 (or C2H4O2.NH3 or C2H7NO2 or CH3COONH4). It is a white solid and can be derived from the reaction of ammonia and acetic acid. It is available commercially and, depending on grade, can be rather inexpensive.
Uses and distinctive properties
As the salt of a weak acid and a weak base, ammonium acetate has a number of distinctive properties.
- NH4C2H3O2 is occasionally employed as a biodegradable de-icing agent.
- It is often used with acetic acid to create a buffer solution, one that can be thermally decomposed to non-ionic products
- Ammonium acetate is useful as a catalyst in the Knoevenagel condensation and as a source of ammonia in the Borch reaction in organic synthesis.
- It is a relatively unusual example of a salt that melts at low temperatures.
- Can be used with distilled water to make a protein precipitating reagent.
- Is often used as an aqueous buffer for ESI mass spectrometry of proteins and other molecules.
- Useful in dialysis as part of a protein purification step to remove contaminants via diffusion.
Ammonium acetate is volatile at low pressures. Because of this, it has been used to replace cell buffers with non-volatile salts in preparing samples for mass spectrometry.[4] It is also popular as a buffer for mobile phases for HPLC with ELSD detection for this reason. Other volatile salts that have been used for this include ammonium formate.
Food Additive
Ammonium acetate is also used as a food additive as an acidity regulator; INS number 264. It is approved for usage in Australia and New Zealand.[5]
Properties
CH3COONH4 is hygroscopic and decomposes easily to acetamide if heated above 165 °C.
- CH3COONH4 → CH3C(O)NH2 + H2O
In this reaction, a salt is converted to two molecular species, which is a relatively uncommon conversion at mild temperatures. Further dehydration leads to acetonitrile or methyl cyanide, an important and widely used solvent.
Production
Ammonium acetate is produced by the neutralization of acetic acid with ammonium carbonate or by saturating glacial acetic acid with dry ammonia gas.[6] Obtaining crystalline ammonium acetate is difficult on account of its aqueous solution giving off ammonia when evaporated.
References
- ↑ 1.0 1.1 1.2 1.3 Pradyot, Patnaik (2003). Handbook of Inorganic Chemicals. The McGraw-Hill Companies, Inc. ISBN 0-07-049439-8.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 http://chemister.ru/Database/properties-en.php?dbid=1&id=354
- ↑ Berman et al., 2008. J Am Soc Mass Spectrom, 19:1230-1236.
- ↑ Australia New Zealand Food Standards Code "Standard 1.2.4 - Labelling of ingredients". Retrieved 2011-10-27.
- ↑ Brannt, William (1914). A practical treatise on the manufacture of vinegar. Lancaster, PA: Henry Carey Baird & Co. pp. 316–317.
Further reading
- G. Jones, Organic Reactions, 1967, volume 15, 204ff (the Knoevenagel Reaction)
External links
| Wikimedia Commons has media related to Ammonium acetate. |
