Ammonia transporter
| Ammonia transporter | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| Symbol | AmtB | ||||||||
| Pfam | PF00909 | ||||||||
| InterPro | IPR001905 | ||||||||
| TCDB | 1.A.11 | ||||||||
| OPM superfamily | 13 | ||||||||
| OPM protein | 2ns1 | ||||||||
| |||||||||
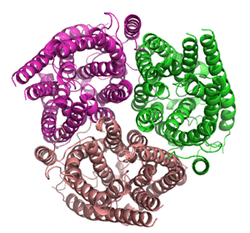
Ammonia transporters are structurally related membrane transport proteins called Amt proteins (ammonia transporters) in bacteria and plants, methylammonium/ammonium permeases (MEPs), in yeast or Rhesus proteins (Rh) in chordates. In humans, the RhAG, RhBG, and RhCG Rhesus proteins constitute solute carrier family 42[2] whilst RhD and RhCE form the Rh blood group system. The three-dimensional structure of the ammonia transport protein AmtB from Escherichia coli has been determined by x-ray crystallography[3][4] revealing a hydrophobic ammonia channel.[5] The human RhCG ammonia transporter was found to have a similar ammonia-conducting channel structure.[1] It was proposed that the erythrocyte Rh complex is a heterotrimer of RhAG, RhD, and RhCE subunits in which RhD and RhCE might play roles in anchoring the ammonia-conducting RhAG subunit to the cytoskeleton. Based on reconstitution experiments, purified RhCG subunits alone can function to transport ammonia.[6] RhCG is required for normal acid excretion by the mouse kidney[7] and epididymis.[8]
Structure
The structure of the ammonia channel from E. coli,[3][4] was, at the time of its publication, the highest resolution structure of any integral membrane protein. It shows a trimer of subunits, each made up of 11 transmembrane helices and containing a pseudo two-fold symmetry.[9] Each monomer contains a hydrophobic ammonia conducting channel. Within the channel are two signature histidine residues which are entirely conserved in the family of active transporters.
While prokaryotic ammonia channel proteins have an N-terminal region which acts as a signal sequence and is cleaved in the mature protein,[10] the Rhesus glycoproteins retain this as a 12th transmembrane helix in the mature protein.[1]
Regulation
In E. coli the AmtB gene is expressed only under limiting nitrogen levels to yield the AmtB protein. It is co-expressed with the GlnK gene which encodes a PII protein. This protein is trimeric also and remains in the cytoplasm.[11] It is covalently modified by a uridyl group. When nitrogen levels outside the cell rise, the ammonia channel must be deactivated to prevent excessive ammonia entering the cell (where ammonia would be combined with glutamate to make glutamine, utilising ATP and thereby depleting the cell's ATP reserves). This deactivation is achieved by de-uridylation of the GlnK protein which then binds to the cytoplasmic face of AmtB and inserts a loop into the ammonia conducting pore. At the tip of this loop is an arginine residue which sterically blocks the channel.[12]
Human ammonia transporter-related proteins
References
- ↑ 1.0 1.1 1.2 Gruswitz, F.; Chaudhary, S.; Ho, J. D.; Schlessinger, A.; Pezeshki, B.; Ho, C. -M.; Sali, A.; Westhoff, C. M.; Stroud, R. M. (2010). "Function of human Rh based on structure of RhCG at 2.1 A". Proceedings of the National Academy of Sciences 107 (21): 9638–9643. doi:10.1073/pnas.1003587107. PMC 2906887. PMID 20457942.
- ↑ Nakhoul, N. L.; Hamm, L. L. (2004). "Non-erythroid Rh glycoproteins: A putative new family of mammalian ammonium transporters". Pflügers Archiv European Journal of Physiology 447 (5): 807–812. doi:10.1007/s00424-003-1142-8. PMID 12920597.
- ↑ 3.0 3.1 1xqe; Khademi, S.; O'Connell j, 3.; Remis, J.; Robles-Colmenares, Y.; Miercke, L. J.; Stroud, R. M. (2004). "Mechanism of Ammonia Transport by Amt/MEP/Rh: Structure of AmtB at 1.35 A". Science 305 (5690): 1587–1594. doi:10.1126/science.1101952. PMID 15361618.
- ↑ 4.0 4.1 2u7c; Zheng, L.; Kostrewa, D.; Bernèche, S.; Winkler, F. K.; Li, X. D. (2004). "The mechanism of ammonia transport based on the crystal structure of AmtB of Escherichia coli". Proceedings of the National Academy of Sciences 101 (49): 17090–17095. doi:10.1073/pnas.0406475101. PMC 535379. PMID 15563598.
- ↑ Khademi, S.; Stroud, R. M. (2006). "The Amt/MEP/Rh Family: Structure of AmtB and the Mechanism of Ammonia Gas Conduction". Physiology 21 (6): 419–429. doi:10.1152/physiol.00051.2005. PMID 17119155.
- ↑ Mouro-Chanteloup, I.; Cochet, S.; Chami, M.; Genetet, S.; Zidi-Yahiaoui, N.; Engel, A.; Colin, Y.; Bertrand, O.; Ripoche, P. (2010). Fatouros, Dimitris, ed. "Functional Reconstitution into Liposomes of Purified Human RhCG Ammonia Channel". PLoS ONE 5 (1): e8921. doi:10.1371/journal.pone.0008921. PMC 2812482. PMID 20126667.
- ↑ Wagner, C. A.; Devuyst, O.; Belge, H.; Bourgeois, S.; Houillier, P. (2010). "The rhesus protein RhCG: A new perspective in ammonium transport and distal urinary acidification". Kidney International 79 (2): 154–161. doi:10.1038/ki.2010.386. PMID 20927037.
- ↑ Biver, S.; Belge, H.; Bourgeois, S.; Van Vooren, P.; Nowik, M.; Scohy, S.; Houillier, P.; Szpirer, J.; Szpirer, C.; Wagner, C. A.; Devuyst, O.; Marini, A. M. (2008). "A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility". Nature 456 (7220): 339–343. doi:10.1038/nature07518. PMID 19020613.
- ↑ Conroy, M. J.; Jamieson, S. J.; Blakey, D.; Kaufmann, T.; Engel, A.; Fotiadis, D.; Merrick, M.; Bullough, P. A. (2004). "Electron and atomic force microscopy of the trimeric ammonium transporter AmtB". EMBO reports 5 (12): 1153–1158. doi:10.1038/sj.embor.7400296. PMC 1299191. PMID 15568015.
- ↑ Thornton, J.; Blakey, D.; Scanlon, E.; Merrick, M. (2006). "The ammonia channel protein AmtB from Escherichia coli is a polytopic membrane protein with a cleavable signal peptide". FEMS Microbiology Letters 258 (1): 114–120. doi:10.1111/j.1574-6968.2006.00202.x. PMID 16630265.
- ↑ Durand, A.; Merrick, M. (2006). "In Vitro Analysis of the Escherichia coli AmtB-GlnK Complex Reveals a Stoichiometric Interaction and Sensitivity to ATP and 2-Oxoglutarate". Journal of Biological Chemistry 281 (40): 29558–29567. doi:10.1074/jbc.M602477200. PMID 16864585.
- ↑ 2nuu; Conroy, M. J.; Durand, A.; Lupo, D.; Li, X. -D.; Bullough, P. A.; Winkler, F. K.; Merrick, M. (2007). "The crystal structure of the Escherichia coli AmtB–GlnK complex reveals how GlnK regulates the ammonia channel". Proceedings of the National Academy of Sciences 104 (4): 1213–1218. doi:10.1073/pnas.0610348104. PMC 1783118. PMID 17220269.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||