Aminodeoxychorismate synthase
In enzymology, an aminodeoxychorismate synthase (EC 2.6.1.85) is an enzyme that catalyzes the chemical reaction
| Aminodeoxychorismate Synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Aminodeoxychorismate synthase | |||||||||
| Identifiers | |||||||||
| EC number | 2.6.1.85 | ||||||||
| CAS number | 132264-37-0 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
chorismate + L-glutamine ---> 4-amino-4-deoxychorismate + L-glutamate
Thus, the two substrates of this enzyme are chorismate and L-glutamine, whereas its two products are 4-amino-4-deoxychorismate and L-glutamate.
It is part of pathway for the biosyntehsis of folates. Folates consist of a family of cofactors that are essential for living organisms. These cofactors are used in one-carbon transfer reactions, which are important in the creation of molecules such as purines, methionine, and thymidylate.[1]
The gene that encodes the 51 kDa protein, aminodeoxychorsimate synthase (PabB), is called pabB.[2]
Nonmenclature
This enzyme belongs to the class of transferase. This means that aminodeoxychorismate synthase catalyzes the transfer of one functional group from a molecule to another. Specifically, in this case as aminodeoxychorismate synthase is a transanimase it transfers an amino group to a keto acid. The systematic name is Chorismate:L-glutamine aminotransferase. The other common names that the enzyme goes by are:[3]
- Aminodeoxychorismate synthase
- ADC synthase
- 4-amino-4-deoxychorismate synthase
- PabB
Reaction
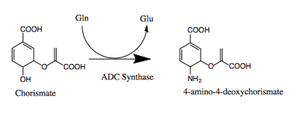
In certain species such as Escherichia coli, aminodeoxychorismate is part of a heterodimeric complex made up of two proteins, Glutamine amidotransferase (PabA) and Aminodeoxychorismate Synthase (PabB). In other species such as plants or lower eukaryote only a single protein is needed.
In Escherichia coli, the reaction is a two step process. Glutamine amidotransferase(PabA) and Aminodeoxychorismate Synthase (PabB) forms a heterodimeric complex that catalyzes the synthesis of 4-amino-4-deoxychorismate which is an important molecule for the synthesis of folic acid. The first step occurs with PabA synthesizing ammonia from glutamine. With both chorismate and ammonia, the second step can occur where PabB uses both substrates to synthesize 4-amino-4-deoxychorsimate. The formation of this hetrodimeric complex occurs best at temperatures around 37 degrees celsius, but when temperatures are below this number the formation of the heterdimeric complex is reduced significantly. Also, when there is certain amount of glutamine such as 5mM, association of PabA and PabB is greatly increased.
In plants such as the Arabidopsis, aminodeoxychorsimate synthase is a monomeric enzyme. The enzyme can catalyze two reactions by first getting glutamine to release ammonia. Once ammonia is released it is able to substitute spots with the OH group at position four on chorismate to synthesize 4-amino-4-deoxychorsimate.[1]
Aminodeoxychorismate can use externally added ammonia as a substrate instead of glutamine. Although it is a very poor substrate when comparied with glutamine which is able to releases ammonia. This is not the same case for Glutamine amidotransferase(PabA). If Glutamine amidotransferase does not have Aminodeoxychorsimate it cannot catalyze ammonia from glutamine.[1]
The kM values for aminodeoxychorsimate is different depending on the species it is from. For E. coli, the kM value is seen to be 13 µM whereas the other Aminosdeoxychorismate synthase's kM value are a lot higher in B. subtilis (410 µM) and S. maltophila (240 µM).[4]
Structure
Aminodeoxychorismate synthase is either a heterodimeric or monomeric enzyme depending on what type of organism it is from. The enzyme has 452-residues and consists of both alpha and beta folds that is very similar to some types of anthranilate synthase. The core of Aminodeoxychorismate consists of two domains that forms a beta sandwich. Also, it has helices and loops around the outside of it's core.[4] The chorsimate binding site on Aminodeoxychorismate synthase consists of amino acids residues that make up beta sheet core and the two key alpha helices.[5]
In some types of Aminodeoxychorismate synthse, it actually has an additional binding site for tryptophan. It is thought to be a nonfunctional vestigal binding site as it is believed that Aminodeoxychorismate synthase evolved from TrpE.[4]
Homologues
Similar structures to Aminodeoxychorismate synthase are:
- Anthranilate synthase
- Isochorismate synthase,
- Salicylate synthase.
A common feature among this list of enzymes is that they all utilize chorismate as a substrate.
Potential as an antifolate target
There has been recent research on developing Aminodeoxychorismate synthase of Stenotrophomonas maltophila as an antifolate target. Strenotrophomonas maltophila infections are fairly uncommon and occurs mostly in hospital settings, but is very hard to cure. Also, Strentrophomonas maltophila infections are often seen in patients with cystic fibrosis, severe immunosupression, and cancer related to hematologic disorders.[5] The reason as to why the infection is so hard to cure is because of the Strenotorphomonas immunity to antibiotics, biofilm and other various reasons. The most common treatment to this type of infection as of now is TMP-SMX, but the drug has potential side effects that includes vomitting, nausea, and allergic concerns. So there is a need for a different treatment that does not have adverse side effects as TMP-SMX. TMP-SMX has shown that by interuptting the folate pathway, Stenotrohomonas maltophila cannot produce the folates that it needs to survive essentially killing the bacteria. Overall, Aminodeoxychorsimate synthase can be a promising target in potential antibiotics by targeting the folate pathway.[5]
Also, it has a poteintal use as a target for new herbicides as Aminodeoxychorsimate sythase is an enzyme that is found only in plants, bacteria, and lower eukaroytes. It is also part of the folate pathway, which is crucial in plants to be able to create.[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 SAHR, Tobias (May 15, 2006). "Folate synthesis in plants: purification, kinetic properties, and inhibition of aminodeoxychorismate synthase". Biochem J. 396(Pt. 1):157-162.
- ↑ 2.0 2.1 Ye, Qi-Zhuang (August 23, 1990). "p-Aminobenzoate synthesis in Escherichia coli: Purificiation and charaterization of PabB as aminodeoxychorsimate synthase and enzyme X as aminodeoxychorsimate lyase". Proc Natl Acad Sci USA.87(23): 9391–9395.
- ↑ "ENZYME: 2.6.1.85". KEGG.
- ↑ 4.0 4.1 4.2 4.3 Parsons, James (November 21, 2001). "Structure of Escherichia coli Aminodeoxychorismate Synthase: Architectural Conservation and Diversity in Chorismate-Utilizing Enzymes". Biochemistry.41 (7), pp 2198–2208.
- ↑ 5.0 5.1 5.2 5.3 Bera, Asim (December 21, 2013). "Structure of Aminodeoxychorismate Synthase from Stenotrophomonas maltophilia". Biochemistry.51(51): 10208–10217.