Amine N-methyltransferase
| amine N-methyltransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 indolethylamine N-methyltransferase (with slight variation on CPK coloration) – See PDB 2A14 | |||||||||
| Identifiers | |||||||||
| EC number | 2.1.1.49 | ||||||||
| CAS number | 51377-47-0 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
In enzymology, an amine N-methyltransferase (EC 2.1.1.49) is an enzyme that is ubiquitously present in non-neural tissues and that catalyzes the N-methylation of tryptamine and structurally related compounds.[1]
The chemical reaction taking place is:
- S-adenosyl-L-methionine + an amine
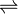 S-adenosyl-L-homocysteine + a methylated amine
S-adenosyl-L-homocysteine + a methylated amine
Thus, the two substrates of this enzyme are S-adenosyl methionine and amine, whereas its two products are S-adenosylhomocysteine and methylated amine. In the case of tryptamine and serotonin these then become the dimethylated indolethylamines dimethyltryptamine (DMT) and bufotenine.[2]
This enzyme belongs to the family of transferases, specifically those transferring one-carbon group methyltransferases. The systematic name of this enzyme class is S-adenosyl-L-methionine:amine N-methyltransferase. Other names in common use include nicotine N-methyltransferase, tryptamine N-methyltransferase, indolethylamine N-methyltransferase, and arylamine N-methyltransferase. This enzyme participates in tryptophan metabolism.
A wide range of primary, secondary and tertiary amines can act as acceptors, including tryptamine, aniline, nicotine and a variety of drugs and other xenobiotics.[1]
Structural studies
As of late 2007, only one structure has been solved for this class of enzymes, with the PDB accession code 2A14.
See also
References
- ↑ 1.0 1.1 tryptamine N-methyltransferase at the US National Library of Medicine Medical Subject Headings (MeSH)
- ↑ J., Kärkkäinen; T. Forsström; J. Tornaeus; K. Wähälä; P. Kiuru; A. Honkanen; U. -H. Stenman; U. Turpeinen; A. Hesso (April 2005). "Potentially hallucinogenic 5-hydroxytryptamine receptor ligands bufotenine and dimethyltryptamine in blood and tissues". Scandinavian Journal of Clinical and Laboratory Investigation 65 (3): 189–199. doi:10.1080/00365510510013604. PMID 16095048. Retrieved October 15, 2008.
- Ansher SS, Jakoby WB; Jakoby (1986). "Amine N-methyltransferases from rabbit liver". J. Biol. Chem. 261 (9): 3996–4001. PMID 3949799.
- Crooks PA, Godin CS, Damani LA, Ansher SS, Jakoby WB; Godin; Damani; Ansher; Jakoby (1988). "Formation of quaternary amines by N-methylation of azaheterocycles with homogeneous amine N-methyltransferases". Biochem. Pharmacol. 37 (9): 1673–7. doi:10.1016/0006-2952(88)90426-1. PMID 3377829.
External links
- EC 2.1.1.49
- Lyon ES, Jakoby WB; Jakoby (1981). "Arylamine N-methyltransferase". Meth. Enzymol. Methods in Enzymology 77: 263–6. doi:10.1016/S0076-6879(81)77035-6. ISBN 9780121819774. PMID 6276654.
- Boarder MR, Rodnight R; Rodnight (1976). "Tryptamine-N-methyltransferase activity in brain tissue: a re-examination". Brain Res. 114 (2): 359–64. doi:10.1016/0006-8993(76)90680-6. PMID 963555.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||