Americium dioxide
| Names | |
|---|---|
| IUPAC name
Americium(IV) oxide | |
| Identifiers | |
| 12005-67-3 | |
| |
| Jmol-3D images | Image |
| |
| Properties | |
| Molecular formula |
AmO2 |
| Molar mass | 275.06 g·mol−1 |
| Appearance | Black crystals |
| Density | 11.68 g/cm3 |
| Structure | |
| Crystal structure | Fluorite (cubic), cF12 |
| Space group | Fm3, No. 225 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Americium dioxide (AmO2) is a black[1] compound of americium. In the solid state AmO2 adopts the fluorite, CaF2 structure.[2] It is used as a source of alpha particles.
Historical Use
Synthesis of americium dioxide involves precipitating a solution of americium in hydrochloric acid (HCl) solution as described by the Oak Ridge National Laboratory[3] The demand for americium dioxide stems from the difficulty of storing the element americium as a liquid in the hydrochloric acid solution because the alpha radiation and hydrochloric acid decomposes storage containers over time. To solve the liquid storage problem, Oak Ridge National Laboratory devised a synthesis to turn liquid americium Hydrochloric acid solution into a precipitated form of americium for safer handling and more efficient storage.[3]
Synthesis (1960)
Synthesis of americium dioxide as described by the Oak Ridge National Laboratory includes making a solution of americium in hydrochloric acid by adding americium to hydrochloric acid, then neutralizing the acid as using ammonium hydroxide (NH4OH) shown in the flow chart.[3]
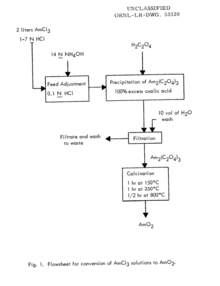
After neutralization using ammonium hydroxide, a saturated oxalic acid solution was added to the now neutralized solution. This causes large americium oxalate crystals to begin to grow; once complete precipitation is achieved, oxalic acid is then added, once again, to attain a slurry. The slurry of americium oxalate and oxalic acid is next agitated before the americium oxalate is filtered out, washed with water, and is partially dried by allowing air to flow through it. Oak Ridge National Laboratory researchers noted that the americium oxalate that was filtered out has a "dusty rose color" appearance.
The americium oxalate is then added to a platinum boat to undergo calcination.The americium oxalate precipitate is dried in a furnace and will begin to decompose at 350 °C. When decomposition begins to occur, the oxalate will turn into the desired black Americium dioxide; to ensure no oxalate remains in the newly forming dioxide, the oven temperature is increased and held at 800 °C then slowly allowed to cool to room temperature.
Americium-aluminium alloys
Americium-aluminium alloys can be formed by melting americium dioxide with aluminium and an additional fluxing agent.[4] The created alloy can undergo neutron irradiation to produce transuranic nuclides. Transuranic meaning an element with a higher atomic number than Uranium (92)[5] and, as described in the toxicology profile, nuclides are elements (and isotopes of elements) that give off radiation and can change into a different isotope or into a different elemental isotope entirely.[6]
References
- ↑ Greenwood, N. N. & Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Pergamon Press. p. 1267. ISBN 0-08-022057-6.
- ↑ Wells, A. F. (Alexander Frank) (1984). Structural inorganic chemistry. Oxford: Clarendon Press. ISBN 978-0-19-855370-0.
- ↑ 3.0 3.1 3.2 "Preparation of Americium Dioxide by Thermal Decomposition of Americium Oxalate in Air" (PDF). Oak Ridge National Laboratory. December 1960. Retrieved 2 May 2013.
- ↑ "Preparation of Americium-Alluminum Alloys". KERNFORSCHUNG GMBH GES FUER. January 1974. Retrieved May 3, 2013.
- ↑ "Transuranic". Oxford Dictionary, 2013. Retrieved May 5, 2013.
- ↑ "Toxicological profile for americium" (PDF). U.S. Department of Health and Human Services. April 2004. Retrieved 15 January 2011.
| ||||||||||