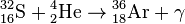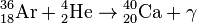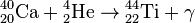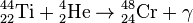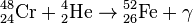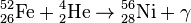Alpha process
The alpha process, also known as the alpha ladder, is one of two classes of nuclear fusion reactions by which stars convert helium into heavier elements, the other being the triple-alpha process.[1] While the triple-alpha process only requires helium, once some carbon is present, other reactions that consume helium are possible:
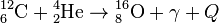 , Q = 7.16 МeV
, Q = 7.16 МeV
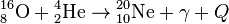 , Q = 4.73 МeV
, Q = 4.73 МeV
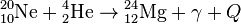 , Q = 9.31 МeV
, Q = 9.31 МeV
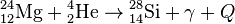 , Q = 9.98 МeV
, Q = 9.98 МeV
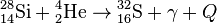 , Q = 6.95 МeV
, Q = 6.95 МeV
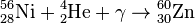 (energy is consumed and the star's core collapses)
(energy is consumed and the star's core collapses)
All these reactions have a very low rate and therefore do not contribute significantly to the energy production in stars; with elements heavier than neon (atomic number > 10), they occur even less easily due to the increasing Coulomb barrier.
Alpha process elements (or alpha elements) are so-called since their most abundant isotopes are integer multiples of four, the mass of the helium nucleus (the alpha particle). Alpha elements are Z ≤ 22: (C, N), O, Ne, Mg, Si, S, Ar, Ca, Ti. They are synthesized by alpha capture prior to the silicon fusing process, a precursor to Type II supernovae. Silicon and calcium are purely alpha process elements. Magnesium can be burned by proton capture reactions. As for oxygen, some authors consider it an alpha element, while others do not. Oxygen is surely an alpha element in low-metallicity population II stars. It is produced in Type II supernovae and its enhancement is well correlated with an enhancement of other alpha process elements. Sometimes carbon and nitrogen are considered alpha process elements, since they are synthesized in nuclear alpha-capture reactions.
The abundance of alpha elements in stars is usually expressed in a logarithmic manner:
![[\alpha/Fe] = \log_{10}{\left(\frac{N_{\alpha}}{N_{Fe}}\right)_{Star}} - \log_{10}{\left(\frac{N_{\alpha}}{N_{Fe}}\right)_{Sun}}](../I/m/b65ee36a95861812c5072ca53c304824.png) ,
,
Here 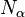 and
and 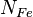 are the number of alpha elements and iron nuclei per unit volume. Theoretical galactic evolution models predict that early in the universe there were more alpha elements relative to iron. Type II supernovae mainly synthesize oxygen and the alpha-elements (Ne, Mg, Si, S, Ar, Ca and Ti) while Type Ia supernovae produce elements of the iron peak (V, Cr, Mn, Fe, Co and Ni).
are the number of alpha elements and iron nuclei per unit volume. Theoretical galactic evolution models predict that early in the universe there were more alpha elements relative to iron. Type II supernovae mainly synthesize oxygen and the alpha-elements (Ne, Mg, Si, S, Ar, Ca and Ti) while Type Ia supernovae produce elements of the iron peak (V, Cr, Mn, Fe, Co and Ni).
References
- ↑ Narlikar, Jayant V (1995). From Black Clouds to Black Holes. World Scientific. ISBN 9810220332.
External links
| ||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||