Allylamine
| | |
 | |
| Names | |
|---|---|
| IUPAC name
3-Amino-prop-1-ene | |
| Other names
3-Aminopropene; 3-Aminopropylene; Monoallylamine; 2-Propenamine; 2-Propen-1-amine; Allyl amine | |
| Identifiers | |
| 107-11-9 | |
| ChEMBL | ChEMBL57286 |
| ChemSpider | 13835977 |
| |
| Jmol-3D images | Image |
| RTECS number | BA5425000 |
| |
| UNII | 48G762T011 |
| Properties | |
| Molecular formula |
C3H7N |
| Molar mass | 57.09 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.7630 g/cm3, liquid |
| Melting point | −88 °C (−126 °F; 185 K) |
| Boiling point | 55 °C (131 °F; 328 K) |
| Acidity (pKa) | 9.49[1] |
| Hazards | |
| Main hazards | Lachrymatory |
| R-phrases | R11 R23/24/25 R51/53 |
| S-phrases | S9 S16 S24/25 S45 S61 |
| NFPA 704 | |
| Flash point | −28 °C (−18 °F; 245 K) |
| 374 °C (705 °F; 647 K) | |
| Explosive limits | 2-22% |
| LD50 (Median lethal dose) |
106 mg/kg |
| Related compounds | |
| Related amine |
Propylamine |
| Related compounds |
Allyl alcohol |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
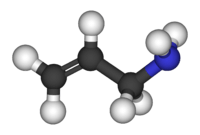
Allylamine is an organic compound with the formula C3H5NH2. This colorless liquid is the simplest stable unsaturated amine.
Production and reactions
All three allylamines, mono-, di-, and triallylamine, are produced by the treating allyl chloride with ammonia followed by distillation.[2] Pure samples can be prepared by hydrolysis of allyl isothiocyanate.[3] It behaves as a typical amine.[4]
Polymerization can be used to prepare the homopolymer (polyallylamine) or copolymers. The polymers are promising membranes for use in reverse osmosis.[2]
Other allylamines
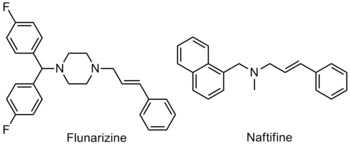
Functionalized allylamines have extensive pharmaceutical applications. Pharmaceutically important allylamines include flunarizine and naftifine. Flunarizine aids in the relief of migraines while naftifine acts to fight common fungus causing infections such as athlete's foot, jock itch, and ringworm.[5]
Safety
Allylamine, like other allyl derivatives is a lachrymator and skin irritant. Its oral LD50 is 106 mg/kg for rats.
References
- ↑ Hall, H.K., J. Am. Chem. Soc., 1957, 79, 5441.
- ↑ 2.0 2.1 Ludger Krähling, Jürgen Krey, Gerald Jakobson, Johann Grolig, Leopold Miksche "Allyl Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a01_425
- ↑ M. T. Leffler (1943). "Allylamine". Org. Synth.; Coll. Vol. 2, p. 24
- ↑ Henk de Koning, W. Nico Speckamp "Allylamine" in Encyclopedia of Reagents for Organic Synthesis, 2001, John Wiley & Sons, Weinheim. doi:10.1002/047084289X.ra043 Article Online Posting Date: April 15, 2001
- ↑ Beck, John F.; Samblanet, Danielle C.; Schmidt, Joseph A. R. (1 January 2013). "Palladium catalyzed intermolecular hydroamination of 1-substituted allenes: an atom-economical method for the synthesis of N-allylamines". RSC Advances 3 (43): 20708. doi:10.1039/c3ra43870h.
