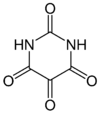
Alloxan
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,3-Diazinane-2,4,5,6-tetrone | |||
| Other names
Mesoxalylurea 5-Oxobarbituric acid | |||
| Identifiers | |||
| 50-71-5 2244-11-3 (Monohydrate) | |||
| ChEBI | CHEBI:76451 | ||
| ChEMBL | ChEMBL1096009 ChEMBL1697709 | ||
| ChemSpider | 5577 | ||
| |||
| Jmol-3D images | Image | ||
| MeSH | Alloxan | ||
| PubChem | 5781 | ||
| |||
| UNII | 6SW5YHA5NG | ||
| Properties | |||
| C4H2N2O4 | |||
| Molar mass | 142.07 g/mol | ||
| Appearance | Solid | ||
| Density | 1.639 g/cm3 | ||
| Melting point | 256 °C (493 °F; 529 K) (decomposition) | ||
| Freely soluble | |||
| Hazards | |||
| MSDS | MSDS | ||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| | |||
| Infobox references | |||
Alloxan (2,4,5,6-pyrimidinetetrone) is an oxygenated pyrimidine derivative. It is present as alloxan hydrate in aqueous solution.
History
Alloxan was originally isolated in 1818 by Brugnatelli and was named in 1838 by Wöhler and Liebig. The name "Alloxan" emerged from an amalgamation of the words "allantoin" and "Oxalsäure" (oxalic acid).
Biological effects
Alloxan is a toxic glucose analogue, which selectively destroys insulin-producing cells in the pancreas (that is beta cells) when administered to rodents and many other animal species. This causes an insulin-dependent diabetes mellitus (called "alloxan diabetes") in these animals, with characteristics similar to type 1 diabetes in humans. Alloxan is selectively toxic to insulin-producing pancreatic beta cells because it preferentially accumulates in beta cells through uptake via the GLUT2 glucose transporter. Alloxan, in the presence of intracellular thiols, generates reactive oxygen species (ROS) in a cyclic reaction with its reduction product, dialuric acid. The beta cell toxic action of alloxan is initiated by free radicals formed in this redox reaction. One study suggests that alloxan does not cause diabetes in humans.[2] Others found a significant difference in alloxan plasma levels in children with and without diabetes Type 1.[3]
Discovery
The compound was discovered by Justus von Liebig and Friedrich Wöhler following the discovery of urea in 1828 and is one of the oldest named organic compounds that exist. The alloxan model of diabetes was first described in rabbits by Dunn, Sheehan and McLetchie in 1943.[4]
Etymology
The name is derived from allantoin, a product of uric acid excreted by the fetus into the allantois, and oxaluric acid derived from oxalic acid and urea, found in urine.
Synthesis
The original preparation for alloxan was by oxidation of uric acid by nitric acid. In another method the monohydrate is prepared by oxidation of barbituric acid by chromium trioxide.[5]
Alloxan is a strong oxidizing agent and it forms a hemiacetal with its reduced reaction product dialuric acid (in which a carbonyl group is reduced to a hydroxyl group) which is called alloxantin.[6]
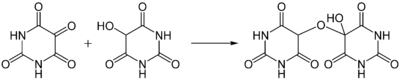 Alloxane (left) with dialuric acid (right) and alloxantin (center)
Alloxane (left) with dialuric acid (right) and alloxantin (center)
Commercial use
Alloxan is a raw material for the production of the purple dye murexide. Carl Wilhelm Scheele discovered the dye in 1776. Murexide is the product of the complex in-situ multistep reaction of alloxantin and gaseous ammonia. Murexide results from the condensation of the unisolated intermediate uramil with alloxan, liberated during the course of the reaction.
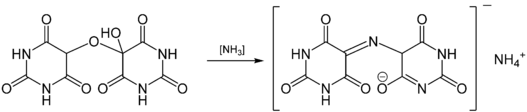 Murexide dye (right) from reaction of alloxantin (left)
Murexide dye (right) from reaction of alloxantin (left)
Scheele sourced uric acid from human calculi (such as kidney stones) and called the compound lithic acid. William Prout investigated the compound in 1818 and he used boa constrictor excrement with up to 90% ammonium acid urate.
Liebig and Wöhler in the nineteenth century coined the name murexide for the dye after the Murex trunculus snail, which is the source of the Tyrian purple of antiquity.
It is also formed as an unintended byproduct in the whitening of maida flour and other flour, but this is said to be a common misconception.
Impact upon beta cells
Because it selectively kills the insulin-producing beta-cells found in the pancreas, alloxan is used to induce diabetes in laboratory animals.[7][8] This occurs most likely because of selective uptake of the compound due to its structural similarity to glucose as well as the beta-cell's highly efficient uptake mechanism (GLUT2).In addition, alloxan has a high affinity to SH-containing cellular compounds and, as a result, reduces glutathione content. Furthermore, alloxan inhibits glucokinase, a SH-containing protein essential for insulin secretion induced by glucose[9]
Some studies have shown that alloxan is not toxic to the human beta-cell, even in very high doses, probably because of differing glucose uptake mechanisms in humans and rodents.[10][11]
Alloxan is, however, toxic to the liver and the kidneys in high doses.
In literature
In the chapter "Nitrogen" of his memoir The Periodic Table, Primo Levi tells of his futile attempt to make alloxan for a cosmetics manufacturer who has read that it can cause permanent reddening of the lips. Levi considers the droppings of pythons as a source for uric acid for making alloxan but he is turned down by the director of the Turin zoo because the zoo already has lucrative contracts with cosmetics companies, so he is obliged to use chickens as his source of uric acid. The synthesis fails, however, "and the alloxan and its resonant name remained a resonant name."[12]
References
- ↑ Merck Index, 11th Edition, 281.
- ↑ Lenzen, S. (2008). "The Mechanisms of Alloxan- and Streptozotocin-induced Diabetes". Diabetologia 51 (2): 216–226. doi:10.1007/s00125-007-0886-7. PMID 18087688.
- ↑ Mrozikiewicz, A.; Kielstrokczewska-Mrozikiewicz, D.; Lstrokowicki, Z.; Chmara, E.; Korzeniowska, K.; Mrozikiewicz, P. M. (1994). "Blood Levels of Alloxan in Children with Insulin-dependent Diabetes Mellitus". Acta Diabetologica 31 (4): 236–237. PMID 7888696.
- ↑ Dunn, J. S.; Sheehan, H. L.; McLetchie, N. G. B. (1943). "Necrosis of Islets of Langerhans Produced Experimentally". Lancet 241 (6242): 484–487. doi:10.1016/S0140-6736(00)42072-6.
- ↑ Holmgren, A. V.; Wenner, W. (1952). "Alloxan monohydrate". Org. Synth. 32: 6.; Coll. Vol. 4, p. 23
- ↑ Tipson, R. S. (1953). "Alloxantin dihydrate". Org. Synth. 33: 3.; Coll. Vol. 4, p. 25
- ↑ Danilova, I.G., Sarapultsev, P.A., Medvedeva, S.U., Gette, I.F., Bulavintceva, T.S. and Sarapultsev, A.P. (2014), Morphological Restructuring of Myocardium During the Early Phase of Experimental Diabetes Mellitus. Anat Rec. doi: 10.1002/ar.23052
- ↑ Loreto D, Elina V. 2009. Experimental surgical models in the laboratory rat. Boca Raton: CRC Press.
- ↑ Szkudelski T. 2001. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 50:536–546
- ↑ Tyrberg, B.; Andersson, A.; Borg, L. A. (2001). "Species Differences in Susceptibility of Transplanted and Cultured Pancreatic Islets to the β-Cell Toxin Alloxan". General and Comparative Endocrinology 122 (3): 238–251. doi:10.1006/gcen.2001.7638. PMID 11356036.
- ↑ Eizirik, D. L.; Pipeleers, D. G.; Ling, Z.; Welsh, N.; Hellerström, C.; Andersson, A. (1994). "Major Species Differences between Humans and Rodents in the Susceptibility to Pancreatic β-Cell Injury". Proceedings of the National Academy of Sciences of the United States of America 91 (20): 9253–9256. doi:10.1073/pnas.91.20.9253. PMC 44790. PMID 7937750.
- ↑ Primo Levi, The Periodic Table (New York: Schocken, 1984), translated by Raymond Rosenthal, 183.
External links
- McLetchie, N. G. (2002). "Alloxan Diabetes, a Discovery, albeit a Minor one" (PDF). Journal of the Royal College of Physicians of Edinburgh 32 (2): 134–142. PMID 12434795.
- The history and chemistry of the Murexide dye