Aldol
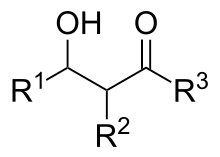
An aldol or aldol adduct (from "Aldehyde alcohol") is a beta-hydroxy ketone or aldehyde, and is the product of aldol addition (as opposed to aldol condensation, which produces an α,β-unsaturated carbonyl moiety).
When used alone, the term "aldol" may also refer specifically to the compound 3-hydroxybutanal.
Discovery
The aldol reaction was discovered in 1872 by French chemist Charles-Adolphe Wurtz. The Wurtz reaction, where two alkyl halides are treated with sodium to form a new carbon−carbon bond, is no longer considered synthetically useful, although the aldol reaction that Wurtz discovered in 1872 has become a staple in organic synthesis.
Alexander Borodin is also credited with the discovery of the aldol reaction together with Wurtz. In 1872 Borodin announced to the Russian Chemical Society the discovery of a new byproduct in aldehyde reactions with properties like that of an alcohol, and he noted similarities with compounds already discussed in publications by Wurtz from the same year.[1]
References
- ↑ McMurry, John (2008). Organic Chemistry, 7th Ed. Thomson Brooks/Cole. pp. 877–80. ISBN 978-0-495-11258-7.