Alachlor
 | |
| Names | |
|---|---|
| IUPAC name
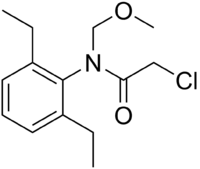
2-Chloro-N-(2,6-diethylphenyl)-N-(methoxymethyl)acetamide | |
| Identifiers | |
| 15972-60-8 | |
| ChEBI | CHEBI:2533 |
| ChEMBL | ChEMBL838038 |
| ChemSpider | 1994 |
| |
| Jmol-3D images | Image Image |
| KEGG | C10928 |
| PubChem | 2078 |
| |
| UNII | 24S2S61PXL |
| Properties | |
| C14H20ClNO2 | |
| Molar mass | 269.767 g/mol |
| Density | 1.12 g/mL |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Alachlor is an herbicide from the chloroacetanilide family. Its mode of action is elongase inhibition, and inhibition of geranylgeranyl pyrophosphate (GGPP) cyclisation enzymes, part of the gibberellin pathway. It is marketed under the trade names Alanex, Bronco, Cannon, Crop Star, Intrro, Lariat, Lasso, Micro-Tech, and Partner.[1] Use of alachlor as a herbicide has been banned in the European Union.
Uses
It is used to control the growth of broad-leafed weeds and grasses in corn and many other crops.
Application details
It mixes well with other herbicides, and is found in mixed formulations with atrazine, glyphosate, trifluralin, and imazaquin. It is used mainly to control annual grasses and broadleaf weeds in corn (maize), soybeans, and peanuts.[2][3]
It is most commonly available as microgranules containing 15% a.i., or emulsifiable concentrate containing 480 g/ litre of a.i. Homologuation in Europe requires a maximum dose of 2,400 g per hectare of a.i., or 5 litres/hectare of emulsifiable concentrate or 17 kg/ha of microgranules. The products are applied as either pre-drilling, soil incorporated, or pre-emergence.[4]
Safety
The United States Environmental Protection Agency classifies the herbicide as toxicity class III - slightly toxic.[5] The EPA has set the Maximum Contaminant Level Goal (MCLG) for Alachlor at zero to prevent long-term effects. The Maximum Contaminant Level (MCL) for drinking water has been set at two parts per billion (2 ppb). The EPA has described the following long-term effects when exposed to levels above the MCL in drinking water exposed to runoff from herbicide used on row crops: slight skin and eye irritation; at lifetime exposure to levels above the MCL: potential damage to liver, kidney, spleen; lining of nose and eyelids; cancer. The major source of environmental release of alachlor is through its manufacture and use as a herbicide. Alachlor was detected in rural domestic well water by EPA's National Survey of Pesticides in Drinking Water Wells. EPA's Pesticides in Ground Water Database reports detections of alachlor in ground water at concentrations above the MCL in at least 15 U.S. states.
Alachlor is a controlled substance under Australian law and is listed as a Schedule 7 (Dangerous Poison) substance. Access, use and storage are strictly controlled under state and territory law. [6]
In January, 2012 a French court found Monsanto, which manufactures Lasso, guilty of chemical poisoning of a French farmer in 2004. Since 2006, use of alachlor as a herbicide has been banned in the European Union.[7]
Environmental properties
Alachlor exhibits moderate sorption in soil, ranging from 43-209 mL/g. Photodegradation is a minor contributor to alachlor fate. Degradation in soil appears to be largely biologically mediated, and produces a number of metabolites. The half life in aerobic soil ranges from about 6 to 15 days, and is considerably shorter under anaerobic conditions.[8] One possible explanation for the short anaerobic half life is the observation that alachlor is rapidly transformed under anoxia to up to 14 degradation products in the presence of iron-bearing ferruginous smectites. The iron in such minerals can be used by certain soil bacteria as an electron acceptor when soils are flooded, thus the process of herbicide transformation by reduced clay is thought to be microbially mediated.[9] Similar observations have been reported for the herbicides trifluralin and atrazine. Alachlor is often found in high school chemistry classrooms to be used as a reactant in demonstrations such as the combustion of magnesium. Alachlor can be used as a substitution for methane gas in such an experiment when gas is not available.[10]
See also
References
- ↑ Arnold P. Appleby, Franz Müller, Serge Carpy “Weed Control“ in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a28_165
- ↑ Cornell Herbicide Profile
- ↑ Oregon State University Pesticide Information Profile
- ↑ "e-phy". Ministère de l'agriculture, de l'agroalimentaire (in French). Retrieved 30 April 2013.
- ↑ Extoxnet Pip - Alachlor
- ↑ http://www.comlaw.gov.au/Details/F2013L01607/Html/Text#_Toc359452381
- ↑ Douet, Marion (13 February 2012). "Monsanto guilty of chemical poisoning in France". Reuters. Retrieved 30 April 2013.
- ↑ Vencill, W.K. 2002. WSSA herbicide handbook (8th edition). Weed Science Society of America. Lawrence, KS, USA. ISBN 1-891276-33-6.
- ↑ Xu, J., J. W. Stucki, J. Wu, J. Kostka, and G. K. Sims. 2001. Fate of atrazine and alachlor in redox-treated ferruginous smectite. Environmental Toxicology & Chemistry 20: 2717-2724.
- ↑ Kappa, Bromide 2013. Substitutions for methane gas in high school combustion demonstrations.
| ||||||||||||||||||||||||||||||||||||||||||