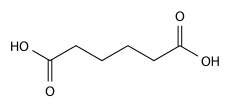
Adipic acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
hexanedioic acid | |
| Other names
hexane-1,6-dicarboxylic acid hexane-1,6-dioic acid | |
| Identifiers | |
| 124-04-9 | |
| ChEBI | CHEBI:30832 |
| ChEMBL | ChEMBL1157 |
| ChemSpider | 191 |
| EC-number | 204-673-3 |
| |
| Jmol-3D images | Image Image |
| KEGG | D08839 |
| PubChem | 196 |
| RTECS number | AU8400000 |
| |
| UNII | 76A0JE0FKJ |
| Properties | |
| Molecular formula |
C6H10O4 |
| Molar mass | 146.14 g·mol−1 |
| Appearance | White crystals[1] |
| Odor | odorless |
| Density | 1.360 g/cm3 |
| Melting point | 152.1 °C (305.8 °F; 425.2 K) |
| Boiling point | 337.5 °C (639.5 °F; 610.6 K) |
| 14 g/L (10 °C) 24 g/L (25 °C) 1600 g/L (100 °C) | |
| Solubility | very soluble in methanol, ethanol soluble in acetone slightly soluble in cyclohexane negligible in benzene, petroleum ether insoluble in acetic acid |
| log P | 0.08 |
| Vapor pressure | 0.0728 Pa (18.5 °C) |
| Acidity (pKa) | 4.43, 5.41 |
| Viscosity | 4.54 cP (160 °C) |
| Structure | |
| Crystal structure | monoclinic |
| Thermochemistry | |
| Std enthalpy of formation (ΔfH |
-1021 KJ/mol |
| Hazards | |
| MSDS | External MSDS |
| EU Index | 607-144-00-9 |
| EU classification | Irritant (Xi) |
| R-phrases | R36 |
| NFPA 704 | |
| Flash point | 196 °C (385 °F; 469 K) |
| 422 °C (792 °F; 695 K) | |
| LD50 (Median lethal dose) |
3600 mg/kg (rat) |
| Related compounds | |
| Related dicarboxylic acids |
glutaric acid pimelic acid |
| Related compounds |
hexanoic acid adipic acid dihydrazide |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Adipic acid is the organic compound with the formula (CH2)4(COOH)2. From an industrial perspective, it is the most important dicarboxylic acid: About 2.5 billion kilograms of this white crystalline powder are produced annually, mainly as a precursor for the production of nylon. Adipic acid otherwise rarely occurs in nature.[2]
Preparation and reactivity
Adipic acid is produced from a mixture of cyclohexanol and cyclohexanone called "KA oil", the abbreviation of ketone-alcohol oil. The KA oil is oxidized with nitric acid to give adipic acid, via a multistep pathway. Early in the reaction the cyclohexanol is converted to the ketone, releasing nitrous acid:
- HOC6H11 + HNO3 → OC6H10 + HNO2 + H2O
Among its many reactions, the cyclohexanone is nitrosated, setting the stage for the scission of the C-C bond:
- HNO2 + HNO3 → NO+NO3− + H2O
- OC6H10 + NO+ → OC6H9-2-NO + H+
Side products of the method include glutaric and succinic acids.[2]
Related processes start from cyclohexanol, which is obtained from the hydrogenation of phenol.[2][3]
Alternative methods of production
Several methods have been developed by carbonylation of butadiene. For example, the hydrocarboxylation proceeds as follows:[2]
- CH2=CH-CH=CH2 + 2 CO + 2 H2O → HO2C(CH2)4CO2H
A method that utilizes principles of green chemistry in that water is the only by-product. Cyclohexene is oxidized with hydrogen peroxide using a tungstate-based catalyst and a phase transfer catalyst.[4] The waste product is water.
Historically, adipic acid was prepared by oxidation of various fats,[5] thus the name (ultimately from Latin adeps, adipis : 'animal fat'; cf. adipose tissue).
Reactions
Adipic acid is a dibasic acid (can be deprotonated twice). Its pKa's are 4.41 and 5.41.[6]
With the carboxylate groups separated by four methylene groups, adipic acid is suited for intramolecular condensation reactions. Upon treatment with barium hydroxide at elevated temperatures, it undergoes ketonization to give cyclopentanone.[7]
Uses
The great majority of the 2.5 billion kg of adipic acid produced annually is used as monomer for the production of nylon by a polycondensation reaction with hexamethylene diamine forming 6,6-nylon. Other major applications also involve polymers: it is a monomer for production of Polyurethane and its esters are plasticizers, especially in PVC.
In medicine
Adipic acid has been incorporated into controlled-release formulation matrix tablets to obtain pH-independent release for both weakly basic and weakly acidic drugs. It has also been incorporated into the polymeric coating of hydrophilic monolithic systems to modulate the intragel pH, resulting in zero-order release of a hydrophilic drug. The disintegration at intestinal pH of the enteric polymer shellac has been reported to improve when adipic acid was used as a pore-forming agent without affecting release in the acidic media. Other controlled-release formulations have included adipic acid with the intention of obtaining a late-burst release profile.[8]
In foods
Small but significant amounts of adipic acid are used as a food ingredient as a flavorant and gelling aid.[9] It is used in some calcium carbonate antacids to make them tart.
Safety
Adipic acid, like most carboxylic acids, is a mild skin irritant. It is mildly toxic, with an LD50 of 3600 mg/kg for oral ingestion by rats.[2]
Environmental
The production of adipic acid is linked to emissions of N
2O,[10] a potent greenhouse gas and cause of stratospheric ozone depletion. At adipic acid producers DuPont and Rhodia (now Invista and Solvay respectively), processes have been implemented to catalytically convert the nitrous oxide to innocuous products:[11]
- 2 N2O → 2 N2 + O2
References
- ↑ Mac Gillavry, C. H. (2010). "The crystal structure of adipic acid". Recueil des Travaux Chimiques des Pays-Bas 60 (8): 605. doi:10.1002/recl.19410600805.
- ↑ 2.0 2.1 2.2 2.3 2.4 Musser, M. T. (2005). "Adipic Acid". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_269.
- ↑ Ellis, B. A. (1925). "Adipic Acid". Org. Synth. 5: 9.; Coll. Vol. 1, p. 560
- ↑ Sato, K.; Aoki, M.; Noyori, R. (1998). "A "Green" route to adipic acid: direct oxidation of cyclohexenes with 30 percent hydrogen peroxide". Science 281 (5383): 1646–47. Bibcode:1998Sci...281.1646S. doi:10.1126/science.281.5383.1646.
- ↑ Ince, Walter (1895). "Preparation of adipic acid and some of its derivatives". Journal Chemical Society, London 67: 155. doi:10.1039/CT8956700155.
- ↑ Cornils, Boy and Lappe, Peter (2006) "Dicarboxylic Acids, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. doi:10.1002/14356007.a08_523
- ↑ Thorpe, J. F. and Kon, G. A. R. (1925). "Cyclopentanone". Org. Synth. 5: 37.; Coll. Vol. 1, p. 192
- ↑ Roew, Raymond (2009), "Adipic Acid", Handbook of Pharmaceutical Excipients, pp. 11–12
- ↑ "Cherry Jell-O Nutrition Facts". Kraft Foods. Retrieved 21 Mar 2012.
- ↑ US EPA. "U.S. Greenhouse Gas Inventory Report, Chapter 4. Industrial Processes" (PDF). Retrieved 2013-11-29.
- ↑ Reimer, R. A.; Slaten, C. S.; Seapan, M.; Koch, T. A. and Triner, V. G. (2000). "Adipic Acid Industry — N2O Abatement". Non-CO2 Greenhouse Gases: Scientific Understanding, Control and Implementation. Netherlands: Springer. pp. 347–358. doi:10.1007/978-94-015-9343-4_56. ISBN 978-94-015-9343-4.
Appendix
- E-number E355.
- U.S. FDA citations – GRAS (21 CFR 184.1009), Indirect additive (21 CFR 175.300, 21 CFR 175.320, 21 CFR 176.170, 21 CFR 176.180, 21 CFR 177.1200, 21 CFR 177.1390, 21 CFR 177.1500, 21 CFR 177.1630, 21 CFR 177.1680, 21 CFR 177.2420, 21 CFR 177.2600)
- European Union Citations – Decision 1999/217/EC – Flavoing Substance; Directive 95/2/EC, Annex IV – Permitted Food Additive; 2002/72/EC, Annex A – Authorized monomer for Food Contact Plastics
