Adderall
 | |
 | |
| Combination of | |
|---|---|
| amphetamine aspartate monohydrate | (25%) stimulant |
| amphetamine sulfate | (25%) stimulant |
| dextroamphetamine saccharate | (25%) stimulant |
| dextroamphetamine sulfate | (25%) stimulant |
| Clinical data | |
| Trade names | Adderall, Adderall XR |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a601234 |
| Licence data | US FDA:link |
| |
| |
|
Physical: none Psychological: moderate | |
| Moderate | |
| Oral, insufflation, rectal, sublingual | |
| Identifiers | |
|
300-62-9 | |
| N06BA02 N06BA01 | |
| PubChem | CID 3007 |
| DrugBank |
DB00182 |
| ChemSpider |
13852819 |
| KEGG |
D03740 |
| ChEBI |
CHEBI:2679 |
| ChEMBL |
CHEMBL405 |
| | |
Adderall[note 1] is a psychostimulant drug of the phenethylamine class used in the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy. Adderall is also used as a performance and cognitive enhancer, and recreationally as an aphrodisiac and euphoriant. The medication is a mixture of various salts of the two amphetamine stereoisomers and inactive ingredients; by salt content, the active ingredients are 75% dextroamphetamine salts (the dextrorotary or "right-handed" enantiomer) and 25% levoamphetamine salts (the levorotary or "left-handed" enantiomer).[note 2][sources 1]
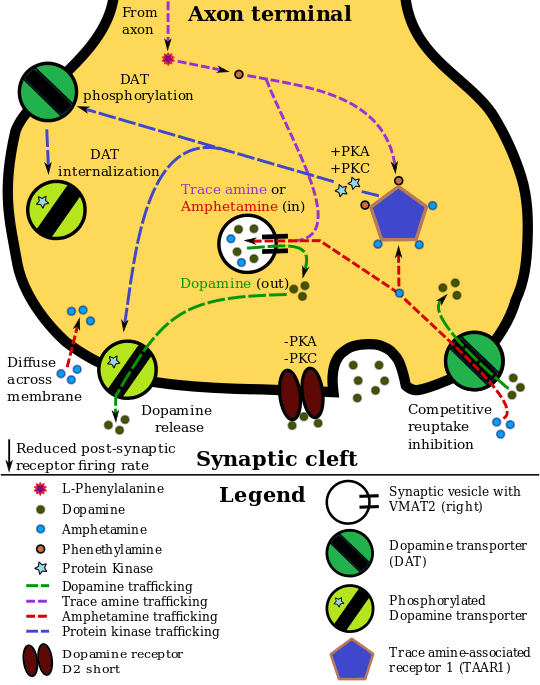
Adderall works by increasing the activity of the neurotransmitters norepinephrine and dopamine in the brain, which results from its interactions with trace amine associated receptor 1 (TAAR1) and vesicular monoamine transporter 2 (VMAT2). Adderall shares many chemical and pharmacological properties with the human trace amine neurotransmitters, especially phenethylamine and N-methylphenethylamine, the latter being an isomer of amphetamine that is produced within the human body.[sources 2]
Adderall is generally well-tolerated and effective in treating the symptoms of ADHD. The most common side effects are cardiovascular, such as irregular heartbeat (usually as a fast heartbeat), and psychological, such as euphoria or anxiety. Much larger doses of Adderall are likely to impair cognitive function and induce rapid muscle breakdown. Drug addiction is a serious risk of Adderall abuse, but only rarely arises from medical use. Very high doses can result in a psychosis (e.g., delusions and paranoia) which rarely occurs at therapeutic doses even during long-term use. Recreational doses are generally much larger than prescribed therapeutic doses, and carry a far greater risk of serious side effects.[sources 3]
Uses
| Grade level | Amphetamine | Adderall |
|---|---|---|
| 8th-graders | 2.60% | 1.80% |
| 10th-graders | 5.90% | 4.40% |
| 12th-graders | 7.90% | 7.40% |

Medical
Adderall is used to treat attention deficit hyperactivity disorder (ADHD) and narcolepsy (a sleep disorder).[18][19] Long-term amphetamine exposure in some animal species is known to produce abnormal dopamine system development or nerve damage,[20][21] but, in humans with ADHD, pharmaceutical amphetamines appear to improve brain development and nerve growth.[22][23][24] Magnetic resonance imaging (MRI) studies suggest that long-term treatment with amphetamine decreases abnormalities in brain structure and function found in subjects with ADHD, and improves function in several parts of the brain, such as the right caudate nucleus of the basal ganglia.[22][23][24]
Reviews of clinical stimulant research have established the safety and effectiveness of long-term amphetamine use for ADHD.[25][26][27] Controlled trials spanning two years have demonstrated treatment effectiveness and safety.[25][27] One review highlighted a nine-month randomized controlled trial in children with ADHD that found an average increase of 4.5 IQ points, continued increases in attention, and continued decreases in disruptive behaviors and hyperactivity.[25]
Current models of ADHD suggest that it is associated with functional impairments in some of the brain's neurotransmitter systems;[5] these functional impairments involve impaired dopamine neurotransmission in the mesocorticolimbic projection and norepinephrine neurotransmission in the locus coeruleus and prefrontal cortex.[5] Psychostimulants like methylphenidate and amphetamine are effective in treating ADHD because they increase neurotransmitter activity in these systems.[11][5][28] Approximately 80% of those who use these stimulants see improvements in ADHD symptoms.[29] Children with ADHD who use stimulant medications generally have better relationships with peers and family members, perform better in school, are less distractible and impulsive, and have longer attention spans.[30][31] The Cochrane Collaboration's review[note 3] on the treatment of adult ADHD with pharmaceutical amphetamines stated that while these drugs improve short-term symptoms, they have higher discontinuation rates than non-stimulant medications due to their adverse side effects.[33]
A Cochrane Collaboration review on the treatment of ADHD in children with tic disorders such as Tourette syndrome indicated that stimulants in general do not make tics worse, but high doses of dextroamphetamine could exacerbate tics in some individuals.[34] Other Cochrane reviews on the use of amphetamine following stroke or acute brain injury indicated that it may improve recovery, but further research is needed to confirm this.[35][36][37]
Dosing and administration
Adderall is available as immediate release tablets or extended-release capsules.[38] The extended release capsule is generally used in the morning.[39] The extended release formulation available under the brand Adderall XR is designed to provide therapeutic effect and plasma concentrations identical to taking two doses 4 hours apart.[40]
Performance-enhancing
A 2015 meta-analysis of high quality clinical trials confirmed that therapeutic doses of amphetamine and methylphenidate result in modest improvements in performance on working memory, episodic memory, and inhibitory control tests in normal healthy adults.[41] Therapeutic doses of amphetamine also enhance cortical network efficiency, an effect which mediates improvements in working memory in all individuals.[11][42] Amphetamine and other ADHD stimulants also improve task saliency (motivation to perform a task) and increase arousal (wakefulness), in turn promoting goal-directed behavior.[11][43][44] Stimulants such as amphetamine can improve performance on difficult and boring tasks and are used by some students as a study and test-taking aid.[11][43][45] Based upon studies of self-reported illicit stimulant use, students primarily use stimulants such as amphetamine for performance enhancement rather than using them as recreational drugs.[46] However, high amphetamine doses that are above the therapeutic range can interfere with working memory and other aspects of cognitive control.[11][43]
Amphetamine is used by some athletes for its psychological and performance-enhancing effects, such as increased stamina and alertness;[47][15] however, its use is prohibited at sporting events regulated by collegiate, national, and international anti-doping agencies.[48][49] In healthy people at oral therapeutic doses, amphetamine has been shown to increase physical strength, acceleration, stamina, and endurance, while reducing reaction time.[47][50][51] Amphetamine improves stamina, endurance, and reaction time primarily through reuptake inhibition and effluxion of dopamine in the central nervous system.[50][51][52] At therapeutic doses, the adverse effects of amphetamine do not impede athletic performance;[47][50][51] however, at much higher doses, amphetamine can induce effects that severely impair performance, such as rapid muscle breakdown and elevated body temperature.[9][10][50]
Adderall has been banned in the National Football League (NFL), Major League Baseball (MLB), National Basketball Association (NBA), and the National Collegiate Athletics Association (NCAA).[53] In leagues such as the NFL, there is a very rigorous process required to obtain an exemption to this rule even when the athlete has been medically prescribed the drug by their physician.[53]
Recreational
Adderall is considered to have a high potential for misuse in a recreational manner.[54][55] Adderall tablets can be crushed and snorted, or dissolved in water and injected.[56] Injection into the bloodstream can be dangerous because insoluble fillers within the tablets can block small blood vessels.[56]
Contraindications
According to the International Programme on Chemical Safety (IPCS) and United States Food and Drug Administration (USFDA),[note 4] amphetamine is contraindicated in people with a history of drug abuse, heart disease, severe agitation, or severe anxiety.[57][58] It is also contraindicated in people currently experiencing arteriosclerosis (hardening of the arteries), glaucoma (increased eye pressure), hyperthyroidism (excessive production of thyroid hormone), or hypertension.[57][58] People who have experienced allergic reactions to other stimulants in the past or who are taking monoamine oxidase inhibitors (MAOIs) are advised not to take amphetamine.[57][58] These agencies also state that anyone with anorexia nervosa, bipolar disorder, depression, hypertension, liver or kidney problems, mania, psychosis, Raynaud's phenomenon, seizures, thyroid problems, tics, or Tourette syndrome should monitor their symptoms while taking amphetamine.[57][58] Evidence from human studies indicates that therapeutic amphetamine use does not cause developmental abnormalities in the fetus or newborns (i.e., it is not a human teratogen), but amphetamine abuse does pose risks to the fetus.[58] Amphetamine has also been shown to pass into breast milk, so the IPCS and USFDA advise mothers to avoid breastfeeding when using it.[57][58] Due to the potential for reversible growth impairments,[note 5] the USFDA advises monitoring the height and weight of children and adolescents prescribed an amphetamine pharmaceutical.[57]
Side effects
The side effects of Adderall are many and varied, but the amount of substance consumed is the primary factor in determining the likelihood and severity of side effects.[9][10][15] Adderall is currently approved for long-term therapeutic use by the USFDA.[10] Recreational use of Adderall generally involves far larger doses and is therefore significantly more dangerous, involving a much greater risk of serious side effects.[15]
Physical
At normal therapeutic doses, the physical side effects of amphetamine vary widely by age and from person to person.[10] Cardiovascular side effects can include hypertension or hypotension from a vasovagal response, Raynaud's phenomenon (reduced blood flow to extremities), and tachycardia (increased heart rate).[10][15][59] Sexual side effects in males may include erectile dysfunction, frequent erections, or prolonged erections.[10] Abdominal side effects may include stomach pain, loss of appetite, nausea, and weight loss.[10] Other potential side effects include acne, blurred vision, dry mouth, excessive grinding of the teeth, profuse sweating, rhinitis medicamentosa (drug-induced nasal congestion), reduced seizure threshold, and tics (a type of movement disorder).[sources 4] Dangerous physical side effects are rare at typical pharmaceutical doses.[15]
Amphetamine stimulates the medullary respiratory centers, producing faster and deeper breaths.[15] In a normal person at therapeutic doses, this effect is usually not noticeable, but when respiration is already compromised, it may be evident.[15] Amphetamine also induces contraction in the urinary bladder sphincter, the muscle which controls urination, which can result in difficulty urinating. This effect can be useful in treating bed wetting and loss of bladder control.[15] The effects of amphetamine on the gastrointestinal tract are unpredictable.[15] If intestinal activity is high, amphetamine may reduce gastrointestinal motility (the rate at which content moves through the digestive system);[15] however, amphetamine may increase motility when the smooth muscle of the tract is relaxed.[15] Amphetamine also has a slight analgesic effect and can enhance the pain relieving effects of opioids.[15]
USFDA-commissioned studies from 2011 indicate that in children, young adults, and adults there is no association between serious adverse cardiovascular events (sudden death, heart attack, and stroke) and the medical use of amphetamine or other ADHD stimulants.[sources 5]
Psychological
Common psychological effects of therapeutic doses can include increased alertness, apprehension, concentration, decreased sense of fatigue, mood swings (elated mood followed by mildly depressed mood), increased initiative, insomnia or wakefulness, self-confidence, and sociability.[10][15] Less common side effects include anxiety, change in libido, grandiosity, irritability, repetitive or obsessive behaviors, and restlessness;[sources 6] these effects depend on the user's personality and current mental state.[15] Amphetamine psychosis (e.g., delusions and paranoia) can occur in heavy users.[9][10][12] Although very rare, this psychosis can also occur at therapeutic doses during long-term therapy.[9][10][13] According to the USFDA, "there is no systematic evidence" that stimulants produce aggressive behavior or hostility.[10]
Amphetamine has also been shown to produce a conditioned place preference in humans taking therapeutic doses,[33][66] meaning that individuals acquire a preference for spending time in places where they have previously used amphetamine.[66][67]
Overdose
An amphetamine overdose can lead to many different symptoms, but is rarely fatal with appropriate care.[58][68] The severity of overdose symptoms increases with dosage and decreases with drug tolerance to amphetamine.[15][58] Tolerant individuals have been known to take as much as 5 grams of amphetamine in a day, which is roughly 100 times the maximum daily therapeutic dose.[58] Symptoms of a moderate and extremely large overdose are listed below; fatal amphetamine poisoning usually also involves convulsions and coma.[9][15] In 2013, overdose on amphetamine, methamphetamine, and other compounds implicated in an "amphetamine use disorder" resulted in an estimated 3,788 deaths worldwide (3,425–4,145 deaths, 95% confidence).[note 6][69]
Pathological overactivation of the mesolimbic pathway, a dopamine pathway that connects the ventral tegmental area to the nucleus accumbens, plays a central role in amphetamine addiction.[70][71] Individuals who frequently overdose on amphetamine during recreational use have a high risk of developing an amphetamine addiction, since repeated overdoses gradually increase the level of accumbal ΔFosB, a "molecular switch" and "master control protein" for addiction.[72][73][74] Once nucleus accumbens ΔFosB is sufficiently overexpressed, it begins to increase the severity of addictive behavior (e.g., compulsive drug-seeking).[72][75] While there are currently no effective drugs for treating amphetamine addiction, regularly engaging in sustained aerobic exercise appears to reduce the risk of developing such an addiction.[76] Sustained aerobic exercise on a regular basis also appears to be an effective treatment for amphetamine addiction;[75][76][77] exercise therapy improves clinical treatment outcomes and may be used as a combination therapy with cognitive behavioral therapy, which is currently the best clinical treatment available.[76][77][78]
| System | Minor or moderate overdose[9][15][58] | Severe overdose[sources 7] |
|---|---|---|
| Cardiovascular |
|
|
| Central nervous system |
|
|
| Musculoskeletal |
| |
| Respiratory |
|
|
| Urinary |
|
|
| Other |
|
Addiction
| Addiction glossary[67][73][81] |
|---|
| • addiction – a state characterized by compulsive engagement in rewarding stimuli, despite adverse consequences |
| • reinforcing stimuli – stimuli that increase the probability of repeating behaviors paired with them |
| • rewarding stimuli – stimuli that the brain interprets as intrinsically positive or as something to be approached |
| • addictive drug – a drug that is both rewarding and reinforcing |
| • addictive behavior – a behavior that is both rewarding and reinforcing |
| • sensitization – an amplified response to a stimulus resulting from repeated exposure to it |
| • drug tolerance – the diminishing effect of a drug resulting from repeated administration at a given dose |
| • drug sensitization or reverse tolerance – the escalating effect of a drug resulting from repeated administration at a given dose |
| • drug dependence – an adaptive state associated with a withdrawal syndrome upon cessation of repeated drug intake |
| • physical dependence – dependence that involves persistent physical–somatic withdrawal symptoms (e.g., fatigue, delirium tremens, and/or persistent insomnia depending on substance) |
| • psychological dependence – dependence that involves emotional–motivational withdrawal symptoms (e.g., dysphoria and anhedonia) |
Addiction is a serious risk with heavy recreational amphetamine use but is unlikely to arise from typical medical use at therapeutic doses.[82][14][15] Drug tolerance develops rapidly in amphetamine abuse (i.e., a recreational amphetamine overdose), so periods of extended use require increasingly larger doses of the drug in order to achieve the same effect.[83][84]
Biomolecular mechanisms
Current models of addiction from chronic drug use involve alterations in gene expression in certain parts of the brain, particularly the nucleus accumbens.[85][86][87] The most important transcription factors[note 7] that produce these alterations are ΔFosB, cAMP response element binding protein (CREB), and nuclear factor kappa B (NFκB).[86] ΔFosB plays a crucial role in the development of drug addictions, since its overexpression in D1-type medium spiny neurons in the nucleus accumbens is necessary and sufficient[note 8] for most of the behavioral and neural adaptations that arise from addiction.[72][73][86] Once ΔFosB is sufficiently overexpressed, it induces an addictive state that becomes increasingly more severe with further increases in ΔFosB expression.[72][73] It has been implicated in addictions to alcohol, cannabinoids, cocaine, nicotine, opioids, phencyclidine, and substituted amphetamines, among others.[75][86][89]
ΔJunD, a transcription factor, and G9a, a histone methyltransferase enzyme, both directly oppose the induction of ΔFosB in the nucleus accumbens (i.e., they oppose increases in its expression).[73][86][90] Sufficiently overexpressing ΔJunD in the nucleus accumbens with viral vectors can completely block many of the neural and behavioral alterations seen in chronic drug abuse (i.e., the alterations mediated by ΔFosB).[86] ΔFosB also plays an important role in regulating behavioral responses to natural rewards, such as palatable food, sex, and exercise.[75][86][91] Since both natural rewards and addictive drugs induce expression of ΔFosB (i.e., they cause the brain to produce more of it), chronic acquisition of these rewards can result in a similar pathological state of addiction.[75][86] Consequently, ΔFosB is the most significant factor involved in both amphetamine addiction and amphetamine-induced sex addictions, which are compulsive sexual behaviors that result from excessive sexual activity and amphetamine use.[75][92] These sex addictions are associated with a dopamine dysregulation syndrome which occurs in some patients taking dopaminergic drugs.[75][91][92]
The effects of amphetamine on gene regulation are both dose- and route-dependent.[87] Most of the research on gene regulation and addiction is based upon animal studies with intravenous amphetamine administration at very high doses.[87] The few studies that have used equivalent (weight-adjusted) human therapeutic doses and oral administration show that these changes, if they occur, are relatively minor.[87] This suggests that medical use of amphetamine does not significantly affect gene regulation.[87]
Pharmacological treatments
As of May 2014, there is no effective pharmacotherapy for amphetamine addiction.[93][94][95] Amphetamine addiction is largely mediated through increased activation of dopamine receptors and co-localized NMDA receptors[note 9] in the nucleus accumbens;[71] magnesium ions inhibit NMDA receptors by blocking the receptor calcium channel.[71][96] One review suggested that, based upon animal testing, pathological (addiction-inducing) amphetamine use significantly reduces the level of intracellular magnesium throughout the brain.[71] Supplemental magnesium[note 10]
Interactions
- Monoamine oxidase inhibitors (MAOIs) taken with Adderall may result in a hypertensive crisis if taken within two weeks after last use of an MAOI type drug.[108]
- Inhibitors of enzymes that directly metabolize amphetamine (particularly FMO3 and CYP2D6) will prolong the elimination of amphetamine.[108][109]
- Stimulants and antidepressants (sedatives and depressants) may increase (decrease) the drug effects of Adderall, and vice versa.[108]
- Dietary pH affects the absorption and elimination half-life of Adderall; an alkaline diet increases the rate of absorption and decreases the rate of excretion, while acidic diets decrease absorption and increase excretion rates.[108]
Pharmacology
Mechanism of action
Amphetamine, the active ingredient of Adderall, works primarily by increasing the activity of the neurotransmitters dopamine and norepinephrine in the brain and more specifically, in the nucleus accumbens, prefrontal cortex, and locus coeruleus regions.[5][28] It also triggers the release of several other neurotransmitters (e.g., serotonin, histamine, and epinephrine, among others) from neurons and also the synthesis of neuropeptides (e.g., cocaine and amphetamine regulated transcript (CART) peptides).[7][110] Both active ingredients of Adderall, dextroamphetamine and levoamphetamine, bind to the same biological targets,[15][111] but their binding affinities (that is, potency) differ somewhat.[15][111] Dextroamphetamine and levoamphetamine are both potent full agonists (activating compounds) of trace amine-associated receptor 1 (TAAR1) and interact with vesicular monoamine transporter 2 (VMAT2), with dextroamphetamine being the more potent agonist of TAAR1.[111] Consequently, dextroamphetamine produces roughly two times more CNS stimulation than levoamphetamine;[111][112] however, levoamphetamine has slightly greater cardiovascular and peripheral effects.[15] Levoamphetamine provides Adderall with a quicker onset and longer-lasting effects than dextroamphetamine alone.[113] It has been reported that certain children have a better clinical response to levoamphetamine.[114][115]
In the absence of amphetamine, VMAT2 will normally move monoamines (e.g., dopamine, histamine, serotonin, norepinephrine, etc.) from the intracellular fluid of a monoamine neuron into its synaptic vesicles, which are essentially chemical storage units inside a neuron.[7] When amphetamine enters a neuron and interacts with VMAT2, the transporter reverses its direction of transport, thereby releasing stored monoamines inside synaptic vesicles back into the neuron's intracellular fluid.[7] Meanwhile, when amphetamine activates TAAR1, the receptor causes the neuron's cell membrane-bound monoamine transporters (i.e., the dopamine transporter, norepinephrine transporter, or serotonin transporter) to either stop transporting molecules altogether (via internalization) or even transport them in reverse;[6] in other words, the reversed membrane transporter will push dopamine, norepinephrine, and serotonin out of the neuron's intracellular fluid and into the synaptic cleft.[6] In summary, by interacting with both VMAT2 and TAAR1, amphetamine releases neurotransmitters from synaptic vesicles (the effect from VMAT2) into the intracellular fluid where they subsequently exit the neuron through the membrane-bound, reversed monoamine transporters (the effect from TAAR1).[6][7]
Pharmacokinetics
The oral bioavailability of amphetamine varies with gastrointestinal pH;[108] it is well absorbed from the gut, and bioavailability is typically over 75% for dextroamphetamine.[116] Amphetamine is a weak base with a pKa of 9–10;[117] consequently, when the pH is basic, more of the drug is in its lipid soluble free base form, and more is absorbed through the lipid-rich cell membranes of the gut epithelium.[117][108] Conversely, an acidic pH means the drug is predominantly in a water soluble cationic (salt) form, and less is absorbed.[117] Approximately 15–40% of amphetamine circulating in the bloodstream is bound to plasma proteins.[118]
The half-life of amphetamine enantiomers differ and vary with urine pH.[117] At normal urine pH, the half-lives of dextroamphetamine and levoamphetamine are 9–11 hours and 11–14 hours, respectively.[117] An acidic diet will reduce the enantiomer half-lives to 8–11 hours; an alkaline diet will increase the range to 16–31 hours.[119][120] The immediate-release and extended release variants of salts of both isomers reach peak plasma concentrations at 3 hours and 7 hours post-dose respectively.[117] Amphetamine is eliminated via the kidneys, with 30–40% of the drug being excreted unchanged at normal urinary pH.[117] When the urinary pH is basic, amphetamine is in its free base form, so less is excreted.[117] When urine pH is abnormal, the urinary recovery of amphetamine may range from a low of 1% to a high of 75%, depending mostly upon whether urine is too basic or acidic, respectively.[117] Amphetamine is usually eliminated within two days of the last oral dose.[119] Apparent half-life and duration of effect increase with repeated use and accumulation of the drug.[121]
CYP2D6, dopamine β-hydroxylase, flavin-containing monooxygenase 3, butyrate-CoA ligase, and glycine N-acyltransferase are the enzymes known to metabolize amphetamine or its metabolites in humans.[sources 8]Related endogenous compounds
Amphetamine has a very similar structure and function to the endogenous trace amines, which are naturally occurring neurotransmitter molecules produced in the human body and brain.[6][8] Among this group, the most closely related compounds are phenethylamine, the parent compound of amphetamine, and N-methylphenethylamine, an isomer of amphetamine (i.e., it has an identical molecular formula).[6][8][132] In humans, phenethylamine is produced directly from L-phenylalanine by the aromatic amino acid decarboxylase (AADC) enzyme, which converts L-DOPA into dopamine as well.[8][132] In turn, N‑methylphenethylamine is metabolized from phenethylamine by phenylethanolamine N-methyltransferase, the same enzyme that metabolizes norepinephrine into epinephrine.[8][132] Like amphetamine, both phenethylamine and N‑methylphenethylamine regulate monoamine neurotransmission via TAAR1;[6][132] unlike amphetamine, both of these substances are broken down by monoamine oxidase B, and therefore have a shorter half-life than amphetamine.[8][132]
History, society, and culture
Richwood Pharmaceuticals, which later merged with Shire plc, introduced the current Adderall brand in 1996 as an instant-release tablet.[133] In 2006, Shire agreed to sell rights to the Adderall name for this instant-release medication to Duramed Pharmaceuticals[134] DuraMed Pharmaceuticals was acquired by Teva Pharmaceuticals in 2008 during their acquisition of Barr Pharmaceuticals, including Barr's Duramed division.[135]
The first generic version of Adderall IR was introduced to market in 2002.[2] Later on, Barr and Shire reached a settlement agreement permitting Barr to offer a generic form of the drug beginning in April 2009.[2][136]
Commercial formulation
Chemically, Adderall is a mixture of several amphetamine salts; specifically, it is composed of equal parts (by mass) of amphetamine aspartate monohydrate, amphetamine sulfate, dextroamphetamine sulfate, and dextroamphetamine saccharate.[40] This drug mixture has slightly stronger CNS effects than racemic amphetamine due to the higher proportion of dextroamphetamine.[6][15] Adderall is produced as both an immediate release (IR) and extended release (XR) formulation.[2][38][137] As of December 2013, ten different companies have produced generic Adderall IR at one point, while Teva Pharmaceutical Industries, Actavis, and Barr Pharmaceuticals currently manufacture generic Adderall XR.[2] Shire plc, the company that held the original patent for Adderall and Adderall XR, still manufactures brand name Adderall XR, but not Adderall IR.[2]
Past formulations
Rexar, a pharmaceutical company, reformulated another drug, branded as Obetrol, and continued to sell this new formulation under the same brand name. This new unapproved formulation was later rebranded and sold as Adderall by Richwood after it acquired Rexar resulting in FDA warning in 1994. Richwood submitted this formulation as NDA 11-522 and Adderall gained FDA approval for the treatment of attention-deficit/hyperactivity disorder on 13 February 1996.[138]
Legal status
- In Canada, amphetamines are in Schedule I of the Controlled Drugs and Substances Act, and can only be obtained by prescription.[139]
- In Japan, the use, production, and import of any medicine containing amphetamine are prohibited.[140]
- In South Korea, amphetamines are prohibited.[141]
- In Thailand, Amphetamines are classified as Type 1 Narcotics.[142]
- In the United Kingdom, amphetamines are regarded as Class B drugs. The maximum penalty for unauthorized possession is five years in prison and an unlimited fine. The maximum penalty for illegal supply is 14 years in prison and an unlimited fine.[143]
- In the United States, amphetamine is a Schedule II prescription drug, classified as a CNS stimulant.[144]
- Internationally (United Nations), amphetamine is in Schedule II of the Convention on Psychotropic Substances.[145][146]
Notes
- ↑ The US nonproprietary name of Adderall is dextroamphetamine sulfate, dextroamphetamine saccharate, amphetamine sulfate and amphetamine aspartate.[1][2] It is sometimes referred to as amphetamine mixed salts and other variants thereof.
- ↑ Enantiomers are molecules that are "mirror images" of one another; they are structurally identical but of the opposite orientation, like left and right hands. The compound "amphetamine" (racemic amphetamine) refers to equal parts of the enantiomers, i.e. 50% levoamphetamine and 50% dextroamphetamine.
- ↑ Cochrane Collaboration reviews are high quality meta-analytic systematic reviews of randomized controlled trials.[32]
- ↑ The statements supported by the USFDA come from prescribing information, which is the copyrighted intellectual property of the manufacturer and approved by the USFDA.
- ↑ In individuals who experience sub-normal height and weight gains, a rebound to normal levels is expected to occur if stimulant therapy is briefly interrupted.[25][27][59] The average reduction in final adult height from continuous stimulant therapy over a 3 year period is 2 cm.[59]
- ↑ The 95% confidence interval indicates that there is a 95% probability that the true number of deaths lies between 3,425 and 4,145.
- ↑ Transcription factors are proteins that increase or decrease the expression of specific genes.[88]
- ↑ In simpler terms, this necessary and sufficient relationship means that ΔFosB overexpression in the nucleus accumbens and addiction-related behavioral and neural adaptations always occur together and never occur alone.
- ↑ NMDA receptors are voltage-dependent ligand-gated ion channels that requires simultaneous binding of glutamate and a co-agonist (D-serine or glycine) to open the ion channel.[96]
- ↑ The review indicated that magnesium L-aspartate and magnesium chloride produce significant changes in addictive behavior;[71] other forms of magnesium were not mentioned. and fluoxetine treatment have been shown to reduce amphetamine self-administration (doses given to oneself) in humans, but neither is an effective monotherapy for amphetamine addiction.[71][97]
Behavioral treatments
Cognitive behavioral therapy is currently the most effective clinical treatment for psychostimulant addiction.[78] Additionally, research on the neurobiological effects of physical exercise suggests that daily aerobic exercise, especially endurance exercise (e.g., marathon running), prevents the development of drug addiction and is an effective adjunct (supplemental) treatment for amphetamine addiction.[75][76][77] Exercise leads to better treatment outcomes when used as an adjunct treatment, particularly for psychostimulant addictions.[76][77] In particular, aerobic exercise decreases psychostimulant self-administration, reduces the reinstatement (i.e., relapse) of drug-seeking, and induces increased dopamine receptor D2 (DRD2) density in the striatum.[75] This is the opposite of pathological stimulant use, which induces decreased striatal DRD2 density.[75]Summary of addiction-related plasticity Form of neural or behavioral plasticity Type of reinforcer Sources Opiates Psychostimulants High fat or sugar food Sexual reward Physical exercise
(aerobic)Environmental
enrichmentΔFosB expression in
nucleus accumbens D1-type MSNs↑ ↑ ↑ ↑ ↑ ↑ [75] Behavioral plasticity Escalation of intake Yes Yes Yes [75] Psychostimulant
cross-sensitizationYes Not applicable Yes Yes Attenuated Attenuated [75] Psychostimulant
self-administration↑ ↑ ↓ ↓ ↓ [75] Psychostimulant
conditioned place preference↑ ↑ ↓ ↑ ↓ ↑ [75] Reinstatement of drug-seeking behavior ↑ ↑ ↓ ↓ [75] Neurochemical plasticity CREB phosphorylation
in the nucleus accumbens↓ ↓ ↓ ↓ ↓ [75] Sensitized dopamine response
in the nucleus accumbensNo Yes No Yes [75] Altered striatal dopamine signaling ↓DRD2, ↑DRD3 ↑DRD1, ↓DRD2, ↑DRD3 ↑DRD1, ↓DRD2, ↑DRD3 ↑DRD2 ↑DRD2 [75] Altered striatal opioid signaling ↑μ-opioid receptors ↑μ-opioid receptors
↑κ-opioid receptors↑μ-opioid receptors ↑μ-opioid receptors No change No change [75] Changes in striatal opioid peptides ↑dynorphin ↑dynorphin ↓enkephalin ↑dynorphin ↑dynorphin [75] Mesocorticolimbic synaptic plasticity Number of dendrites in the nucleus accumbens ↓ ↑ ↑ [75] Dendritic spine density in
the nucleus accumbens↓ ↑ No change ↑ [75] Dependence and withdrawal
According to another Cochrane Collaboration review on withdrawal in individuals who compulsively use amphetamine and methamphetamine, "when chronic heavy users abruptly discontinue amphetamine use, many report a time-limited withdrawal syndrome that occurs within 24 hours of their last dose."[98] This review noted that withdrawal symptoms in chronic, high-dose users are frequent, occurring in up to 87.6% of cases, and persist for three to four weeks with a marked "crash" phase occurring during the first week.[98] Amphetamine withdrawal symptoms can include anxiety, drug craving, depressed mood, fatigue, increased appetite, increased movement or decreased movement, lack of motivation, sleeplessness or sleepiness, and lucid dreams.[98] The review indicated that withdrawal symptoms are associated with the degree of dependence, suggesting that therapeutic use would result in far milder discontinuation symptoms.[98] Manufacturer prescribing information does not indicate the presence of withdrawal symptoms following discontinuation of amphetamine use after an extended period at therapeutic doses.[99][100][101]Toxicity and psychosis
See also: Stimulant psychosisIn rodents and primates, sufficiently high doses of amphetamine cause dopaminergic neurotoxicity, or damage to dopamine neurons, which is characterized by reduced transporter and receptor function.[102] There is no evidence that amphetamine is directly neurotoxic in humans.[103][104] However, large doses of amphetamine may cause indirect neurotoxicity as a result of increased oxidative stress from reactive oxygen species and autoxidation of dopamine.[20][105][106] A severe amphetamine overdose can result in a stimulant psychosis that may involve a variety of symptoms, such as paranoia and delusions.[12] A Cochrane Collaboration review on treatment for amphetamine, dextroamphetamine, and methamphetamine psychosis states that about 5–15% of users fail to recover completely.[12][107] According to the same review, there is at least one trial that shows antipsychotic medications effectively resolve the symptoms of acute amphetamine psychosis.[12] Psychosis very rarely arises from therapeutic use.[13]"Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. pp. 4–6. Retrieved 30 December 2013.
Reference notes
- ↑ [1][3][4]
- ↑ [5][6][7][8]
- ↑ [9][10][11][12][13][14][15][16]
- ↑ [10][15][59][60]
- ↑ [61][62][63][64]
- ↑ [3][10][15][65]
- ↑ [79][9][15][68][80]
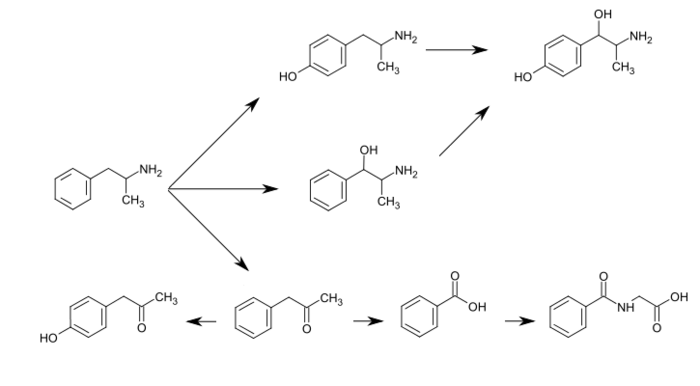
- ↑ [117][122][123][124][109][125][126][127]#Amphe Amphetamine has a variety of excreted metabolic products, including 4-hydroxyamfetamine, 4-hydroxynorephedrine, 4-hydroxyphenylacetone, benzoic acid, hippuric acid, norephedrine, and phenylacetone.[117][119][128] Among these metabolites, the active sympathomimetics are 4‑hydroxyamphetamine,[129] 4‑hydroxynorephedrine,[130] and norephedrine.[131] The main metabolic pathways involve aromatic para-hydroxylation, aliphatic alpha- and beta-hydroxylation, N-oxidation, N-dealkylation, and deamination.[117][119] The known pathways and detectable metabolites in humans include the following:[117][109][128]
Metabolic pathways of amphetamineThe primary active metabolites of amphetamine are 4-hydroxyamphetamine and norephedrine;[128] at normal urine pH, about 30–40% of amphetamine is excreted unchanged and roughly 50% is excreted as the inactive metabolites (bottom row).[117] The remaining 10–20% is excreted as the active metabolites.[117] Benzoic acid is metabolized by butyrate-CoA ligase into an intermediate product, benzoyl-CoA,[126] which is then metabolized by glycine N-acyltransferase into hippuric acid.kaline diet increases"Substrate/Product". glycine N-acyltransferase. BRENDA. Technische Universität Braunschweig. Retrieved 7 May 2014.
References
- ↑ 1.0 1.1 Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present – a pharmacological and clinical perspective". J. Psychopharmacol. 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
Mixed enantiomers/mixed salts amphetamine (3:1 d:l isomers)
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "National Drug Code Amphetamine Search Results". National Drug Code Directory. United States Food and Drug Administration. Archived from the original on 7 February 2014. Retrieved 16 December 2013.
- ↑ 3.0 3.1 Montgomery KA (June 2008). "Sexual desire disorders". Psychiatry (Edgmont) 5 (6): 50–55. PMC 2695750. PMID 19727285.
- ↑ Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, Utzinger L, Fusillo S (January 2008). "Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature". J. Am. Acad. Child Adolesc. Psychiatry 47 (1): 21–31. doi:10.1097/chi.0b013e31815a56f1. PMID 18174822.
Stimulant misuse appears to occur both for performance enhancement and their euphorogenic effects, the latter being related to the intrinsic properties of the stimulants (e.g., IR versus ER profile) ...
Although useful in the treatment of ADHD, stimulants are controlled II substances with a history of preclinical and human studies showing potential abuse liability. - ↑ 5.0 5.1 5.2 5.3 5.4 Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. pp. 154–157. ISBN 9780071481274.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 Miller GM (January 2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". J. Neurochem. 116 (2): 164–76. doi:10.1111/j.1471-4159.2010.07109.x. PMC 3005101. PMID 21073468.
- ↑ 7.0 7.1 7.2 7.3 7.4 Eiden LE, Weihe E (January 2011). "VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse". Ann. N. Y. Acad. Sci. 1216: 86–98. doi:10.1111/j.1749-6632.2010.05906.x. PMID 21272013.
VMAT2 is the CNS vesicular transporter for not only the biogenic amines DA, NE, EPI, 5-HT, and HIS, but likely also for the trace amines TYR, PEA, and thyronamine (THYR) ... [Trace aminergic] neurons in mammalian CNS would be identifiable as neurons expressing VMAT2 for storage, and the biosynthetic enzyme aromatic amino acid decarboxylase (AADC).
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacol. Ther. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 9.7 "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. December 2013. p. 11. Retrieved 30 December 2013.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 10.7 10.8 10.9 10.10 10.11 10.12 10.13 "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. December 2013. pp. 4–8. Retrieved 30 December 2013.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 13: Higher Cognitive Function and Behavioral Control". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. p. 318. ISBN 9780071481274.
- ↑ 12.0 12.1 12.2 12.3 12.4 Shoptaw SJ, Kao U, Ling W (January 2009). Shoptaw SJ, Ali R, ed. "Treatment for amphetamine psychosis". Cochrane Database Syst. Rev. (1): CD003026. doi:10.1002/14651858.CD003026.pub3. PMID 19160215.
A minority of individuals who use amphetamines develop full-blown psychosis requiring care at emergency departments or psychiatric hospitals. In such cases, symptoms of amphetamine psychosis commonly include paranoid and persecutory delusions as well as auditory and visual hallucinations in the presence of extreme agitation. More common (about 18%) is for frequent amphetamine users to report psychotic symptoms that are sub-clinical and that do not require high-intensity intervention ...
About 5–15% of the users who develop an amphetamine psychosis fail to recover completely (Hofmann 1983) ...
Findings from one trial indicate use of antipsychotic medications effectively resolves symptoms of acute amphetamine psychosis. - ↑ 13.0 13.1 13.2 Greydanus D. "Stimulant Misuse: Strategies to Manage a Growing Problem" (PDF). American College Health Association (Review Article). ACHA Professional Development Program. p. 20. Retrieved 2 November 2013.
- ↑ 14.0 14.1 Stolerman IP (2010). Stolerman IP, ed. Encyclopedia of Psychopharmacology. Berlin, Germany; London, England: Springer. p. 78. ISBN 9783540686989.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 15.6 15.7 15.8 15.9 15.10 15.11 15.12 15.13 15.14 15.15 15.16 15.17 15.18 15.19 15.20 15.21 15.22 15.23 15.24 15.25 Westfall DP, Westfall TC (2010). "Miscellaneous Sympathomimetic Agonists". In Brunton LL, Chabner BA, Knollmann BC. Goodman & Gilman's Pharmacological Basis of Therapeutics (12th ed.). New York, USA: McGraw-Hill. ISBN 9780071624428.
- ↑ Cooper, WO; Habel, LA; Sox, CM; Chan, KA; Arbogast, PG; Cheetham, TC; Murray, KT; Quinn, VP; Stein, CM; Callahan, ST; Fireman, BH; Fish, FA; Kirshner, HS; O'Duffy, A; Connell, FA; Ray, WA (17 November 2011). "ADHD drugs and serious cardiovascular events in children and young adults.". The New England Journal of Medicine 365 (20): 1896–904. doi:10.1056/NEJMoa1110212. PMID 22043968.
- ↑ "Prescription Drugs". National Institute on Drug Abuse. Archived from the original on 4 April 2014. Retrieved 23 May 2014.
- ↑ "Adderall IR Prescribing Information" (PDF). United States Food and Drug Administration. Barr Laboratories, Inc. March 2007. pp. 4–5. Retrieved 2 November 2013.
- ↑ Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present – a pharmacological and clinical perspective". J. Psychopharmacol. 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
- ↑ 20.0 20.1 Carvalho M, Carmo H, Costa VM, Capela JP, Pontes H, Remião F, Carvalho F, Bastos Mde L (August 2012). "Toxicity of amphetamines: an update". Arch. Toxicol. 86 (8): 1167–1231. doi:10.1007/s00204-012-0815-5. PMID 22392347.
- ↑ Berman S, O'Neill J, Fears S, Bartzokis G, London ED (October 2008). "Abuse of amphetamines and structural abnormalities in the brain". Ann. N. Y. Acad. Sci. 1141: 195–220. doi:10.1196/annals.1441.031. PMC 2769923. PMID 18991959.
- ↑ 22.0 22.1 Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K (February 2013). "Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects". JAMA Psychiatry 70 (2): 185–198. doi:10.1001/jamapsychiatry.2013.277. PMID 23247506.
- ↑ 23.0 23.1 Spencer TJ, Brown A, Seidman LJ, Valera EM, Makris N, Lomedico A, Faraone SV, Biederman J (September 2013). "Effect of psychostimulants on brain structure and function in ADHD: a qualitative literature review of magnetic resonance imaging-based neuroimaging studies". J. Clin. Psychiatry 74 (9): 902–917. doi:10.4088/JCP.12r08287. PMC 3801446. PMID 24107764.
- ↑ 24.0 24.1 Frodl T, Skokauskas N (February 2012). "Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects.". Acta psychiatrica Scand. 125 (2): 114–126. doi:10.1111/j.1600-0447.2011.01786.x. PMID 22118249.
- ↑ 25.0 25.1 25.2 25.3 Millichap JG (2010). "Chapter 3: Medications for ADHD". In Millichap JG. Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD (2nd ed.). New York, USA: Springer. pp. 121–123, 125–127. ISBN 9781441913968.
Ongoing research has provided answers to many of the parents’ concerns, and has confirmed the effectiveness and safety of the long-term use of medication.
- ↑ Arnold LE, Hodgkins P, Caci H, Kahle J, Young S (February 2015). "Effect of treatment modality on long-term outcomes in attention-deficit/hyperactivity disorder: a systematic review". PLoS ONE 10 (2): e0116407. doi:10.1371/journal.pone.0116407. PMC 4340791. PMID 25714373.
The highest proportion of improved outcomes was reported with combination treatment (83% of outcomes). Among significantly improved outcomes, the largest effect sizes were found for combination treatment. The greatest improvements were associated with academic, self-esteem, or social function outcomes.
- ↑ 27.0 27.1 27.2 Huang YS, Tsai MH (July 2011). "Long-term outcomes with medications for attention-deficit hyperactivity disorder: current status of knowledge". CNS Drugs 25 (7): 539–554. doi:10.2165/11589380-000000000-00000. PMID 21699268.
Recent studies have demonstrated that stimulants, along with the non-stimulants atomoxetine and extended-release guanfacine, are continuously effective for more than 2-year treatment periods with few and tolerable adverse effects.
- ↑ 28.0 28.1 Bidwell LC, McClernon FJ, Kollins SH (August 2011). "Cognitive enhancers for the treatment of ADHD". Pharmacol. Biochem. Behav. 99 (2): 262–274. doi:10.1016/j.pbb.2011.05.002. PMC 3353150. PMID 21596055.
- ↑ Parker J, Wales G, Chalhoub N, Harpin V (September 2013). "The long-term outcomes of interventions for the management of attention-deficit hyperactivity disorder in children and adolescents: a systematic review of randomized controlled trials". Psychol. Res. Behav. Manag. 6: 87–99. doi:10.2147/PRBM.S49114. PMC 3785407. PMID 24082796.
Only one paper53 examining outcomes beyond 36 months met the review criteria. ... There is high level evidence suggesting that pharmacological treatment can have a major beneficial effect on the core symptoms of ADHD (hyperactivity, inattention, and impulsivity) in approximately 80% of cases compared with placebo controls, in the short term.
- ↑ Millichap JG (2010). "Chapter 3: Medications for ADHD". In Millichap JG. Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD (2nd ed.). New York, USA: Springer. pp. 111–113. ISBN 9781441913968.
- ↑ "Stimulants for Attention Deficit Hyperactivity Disorder". WebMD. Healthwise. 12 April 2010. Retrieved 12 November 2013.
- ↑ Scholten RJ, Clarke M, Hetherington J (August 2005). "The Cochrane Collaboration". Eur. J. Clin. Nutr. 59 Suppl 1: S147–S149; discussion S195–S196. doi:10.1038/sj.ejcn.1602188. PMID 16052183.
- ↑ 33.0 33.1 Castells X, Ramos-Quiroga JA, Bosch R, Nogueira M, Casas M (June 2011). Castells X, ed. "Amphetamines for Attention Deficit Hyperactivity Disorder (ADHD) in adults". Cochrane Database Syst. Rev. (6): CD007813. doi:10.1002/14651858.CD007813.pub2. PMID 21678370.
- ↑ Pringsheim T, Steeves T (April 2011). Pringsheim T, ed. "Pharmacological treatment for Attention Deficit Hyperactivity Disorder (ADHD) in children with comorbid tic disorders". Cochrane Database Syst. Rev. (4): CD007990. doi:10.1002/14651858.CD007990.pub2. PMID 21491404.
- ↑ Martinsson L, Hårdemark H, Eksborg S (January 2007). Martinsson L, ed. "Amphetamines for improving recovery after stroke". Cochrane Database Syst. Rev. (1): CD002090. doi:10.1002/14651858.CD002090.pub2. PMID 17253474.
- ↑ Forsyth RJ, Jayamoni B, Paine TC (October 2006). Forsyth RJ, ed. "Monoaminergic agonists for acute traumatic brain injury". Cochrane Database Syst. Rev. (4): CD003984. doi:10.1002/14651858.CD003984.pub2. PMID 17054192.
- ↑ Harbeck-Seu A, Brunk I, Platz T, Vajkoczy P, Endres M, Spies C (April 2011). "A speedy recovery: amphetamines and other therapeutics that might impact the recovery from brain injury". Curr. Opin. Anaesthesiol. 24 (2): 144–153. doi:10.1097/ACO.0b013e328344587f. PMID 21386667.
- ↑ 38.0 38.1 "ADDERALL (CII)" (PDF). Food and Drug Administration. February 2007. Retrieved 23 June 2009.
- ↑ "Amphetamine/Dextroamphetamine (by mouth)". Micromedex consumer medication information. Truven Health Analytics. Retrieved 20 June 2013.
- ↑ 40.0 40.1 "Medication Guide Adderall XR" (PDF). US Food and Drug Administration (FDA). Retrieved 19 May 2013.
- ↑ Ilieva IP, Hook CJ, Farah MJ (January 2015). "Prescription Stimulants' Effects on Healthy Inhibitory Control, Working Memory, and Episodic Memory: A Meta-analysis". J. Cogn. Neurosci.: 1–21. doi:10.1162/jocn_a_00776. PMID 25591060.
- ↑ Devous MD, Trivedi MH, Rush AJ (April 2001). "Regional cerebral blood flow response to oral amphetamine challenge in healthy volunteers". J. Nucl. Med. 42 (4): 535–542. PMID 11337538.
- ↑ 43.0 43.1 43.2 Wood S, Sage JR, Shuman T, Anagnostaras SG (January 2014). "Psychostimulants and cognition: a continuum of behavioral and cognitive activation". Pharmacol. Rev. 66 (1): 193–221. doi:10.1124/pr.112.007054. PMID 24344115.
- ↑ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 10: Neural and Neuroendocrine Control of the Internal Milieu". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. p. 266. ISBN 9780071481274.
Dopamine acts in the nucleus accumbens to attach motivational significance to stimuli associated with reward.
- ↑ Twohey M (26 March 2006). "Pills become an addictive study aid". JS Online. Archived from the original on 15 August 2007. Retrieved 2 December 2007.
- ↑ Teter CJ, McCabe SE, LaGrange K, Cranford JA, Boyd CJ (October 2006). "Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration". Pharmacotherapy 26 (10): 1501–1510. doi:10.1592/phco.26.10.1501. PMC 1794223. PMID 16999660.
- ↑ 47.0 47.1 47.2 Liddle DG, Connor DJ (June 2013). "Nutritional supplements and ergogenic AIDS". Prim. Care 40 (2): 487–505. doi:10.1016/j.pop.2013.02.009. PMID 23668655.
Amphetamines and caffeine are stimulants that increase alertness, improve focus, decrease reaction time, and delay fatigue, allowing for an increased intensity and duration of training ...
Physiologic and performance effects
• Amphetamines increase dopamine/norepinephrine release and inhibit their reuptake, leading to central nervous system (CNS) stimulation
• Amphetamines seem to enhance athletic performance in anaerobic conditions 39 40
• Improved reaction time
• Increased muscle strength and delayed muscle fatigue
• Increased acceleration
• Increased alertness and attention to task - ↑ Bracken NM (January 2012). "National Study of Substance Use Trends Among NCAA College Student-Athletes" (PDF). NCAA Publications. National Collegiate Athletic Association. Retrieved 8 October 2013.
- ↑ Docherty JR (June 2008). "Pharmacology of stimulants prohibited by the World Anti-Doping Agency (WADA)". Br. J. Pharmacol. 154 (3): 606–622. doi:10.1038/bjp.2008.124. PMC 2439527. PMID 18500382.
- ↑ 50.0 50.1 50.2 50.3 Parr JW (July 2011). "Attention-deficit hyperactivity disorder and the athlete: new advances and understanding". Clin. Sports Med. 30 (3): 591–610. doi:10.1016/j.csm.2011.03.007. PMID 21658550.
- ↑ 51.0 51.1 51.2 Roelands B, de Koning J, Foster C, Hettinga F, Meeusen R (May 2013). "Neurophysiological determinants of theoretical concepts and mechanisms involved in pacing". Sports Med. 43 (5): 301–311. doi:10.1007/s40279-013-0030-4. PMID 23456493.
- ↑ Parker KL, Lamichhane D, Caetano MS, Narayanan NS (October 2013). "Executive dysfunction in Parkinson's disease and timing deficits". Front. Integr. Neurosci. 7: 75. doi:10.3389/fnint.2013.00075. PMC 3813949. PMID 24198770.
Manipulations of dopaminergic signaling profoundly influence interval timing, leading to the hypothesis that dopamine influences internal pacemaker, or “clock,” activity. For instance, amphetamine, which increases concentrations of dopamine at the synaptic cleft advances the start of responding during interval timing, whereas antagonists of D2 type dopamine receptors typically slow timing;... Depletion of dopamine in healthy volunteers impairs timing, while amphetamine releases synaptic dopamine and speeds up timing.
- ↑ 53.0 53.1 Leon Moore, David. "Do pro sports leagues have an Adderall problem?". USA TODAY. Retrieved 4 May 2014.
- ↑ "Commonly Abused Prescription Drugs Chart". National Institute on Drug Abuse. Retrieved 7 May 2012.
- ↑ "Stimulant ADHD Medications – Methylphenidate and Amphetamines". National Institute on Drug Abuse,. Retrieved 7 May 2012.
- ↑ 56.0 56.1 "National Institute on Drug Abuse. 2009. Stimulant ADHD Medications – Methylphenidate and Amphetamines". National Institute on Drug Abuse. Retrieved 27 February 2013.
- ↑ 57.0 57.1 57.2 57.3 57.4 57.5
- ↑ 58.0 58.1 58.2 58.3 58.4 58.5 58.6 58.7 58.8 58.9 Heedes G; Ailakis J. "Amphetamine (PIM 934)". INCHEM. International Programme on Chemical Safety. Retrieved 24 June 2014.
- ↑ 59.0 59.1 59.2 59.3 Vitiello B (April 2008). "Understanding the risk of using medications for attention deficit hyperactivity disorder with respect to physical growth and cardiovascular function". Child Adolesc. Psychiatr. Clin. N. Am. 17 (2): 459–474. doi:10.1016/j.chc.2007.11.010. PMC 2408826. PMID 18295156.
- ↑ Ramey JT, Bailen E, Lockey RF (2006). "Rhinitis medicamentosa" (PDF). J. Investig. Allergol. Clin. Immunol. 16 (3): 148–155. PMID 16784007. Retrieved 29 April 2015.
Table 2. Decongestants Causing Rhinitis Medicamentosa
– Nasal decongestants:
– Sympathomimetic:
• Amphetamine - ↑ "FDA Drug Safety Communication: Safety Review Update of Medications used to treat Attention-Deficit/Hyperactivity Disorder (ADHD) in children and young adults". United States Food and Drug Administration. 20 December 2011. Retrieved 4 November 2013.
- ↑ Cooper WO, Habel LA, Sox CM, Chan KA, Arbogast PG, Cheetham TC, Murray KT, Quinn VP, Stein CM, Callahan ST, Fireman BH, Fish FA, Kirshner HS, O'Duffy A, Connell FA, Ray WA (November 2011). "ADHD drugs and serious cardiovascular events in children and young adults". N. Engl. J. Med. 365 (20): 1896–1904. doi:10.1056/NEJMoa1110212. PMID 22043968.
- ↑ "FDA Drug Safety Communication: Safety Review Update of Medications used to treat Attention-Deficit/Hyperactivity Disorder (ADHD) in adults". United States Food and Drug Administration. 15 December 2011. Retrieved 4 November 2013.
- ↑ Habel LA, Cooper WO, Sox CM, Chan KA, Fireman BH, Arbogast PG, Cheetham TC, Quinn VP, Dublin S, Boudreau DM, Andrade SE, Pawloski PA, Raebel MA, Smith DH, Achacoso N, Uratsu C, Go AS, Sidney S, Nguyen-Huynh MN, Ray WA, Selby JV (December 2011). "ADHD medications and risk of serious cardiovascular events in young and middle-aged adults". JAMA 306 (24): 2673–2683. doi:10.1001/jama.2011.1830. PMC 3350308. PMID 22161946.
- ↑ O'Connor PG (February 2012). "Amphetamines". Merck Manual for Health Care Professionals. Merck. Retrieved 8 May 2012.
- ↑ 66.0 66.1 Childs E, de Wit H (May 2009). "Amphetamine-induced place preference in humans". Biol. Psychiatry 65 (10): 900–904. doi:10.1016/j.biopsych.2008.11.016. PMC 2693956. PMID 19111278.
This study demonstrates that humans, like nonhumans, prefer a place associated with amphetamine administration. These findings support the idea that subjective responses to a drug contribute to its ability to establish place conditioning.
- ↑ 67.0 67.1 Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and Addictive Disorders". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 364–375. ISBN 9780071481274.
- ↑ 68.0 68.1 Spiller HA, Hays HL, Aleguas A (June 2013). "Overdose of drugs for attention-deficit hyperactivity disorder: clinical presentation, mechanisms of toxicity, and management". CNS Drugs 27 (7): 531–543. doi:10.1007/s40263-013-0084-8. PMID 23757186.
Amphetamine, dextroamphetamine, and methylphenidate act as substrates for the cellular monoamine transporter, especially the dopamine transporter (DAT) and less so the norepinephrine (NET) and serotonin transporter. The mechanism of toxicity is primarily related to excessive extracellular dopamine, norepinephrine, and serotonin.
- ↑ Collaborators (2015). "Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013" (PDF). Lancet 385 (9963): 117–171. doi:10.1016/S0140-6736(14)61682-2. PMC 4340604. PMID 25530442. Retrieved 3 March 2015.
Amphetamine use disorders ... 3,788 (3,425–4,145)
- ↑ Kanehisa Laboratories (10 October 2014). "Amphetamine – Homo sapiens (human)". KEGG Pathway. Retrieved 31 October 2014.
- ↑ 71.0 71.1 71.2 71.3 71.4 71.5 Nechifor M (March 2008). "Magnesium in drug dependences". Magnes. Res. 21 (1): 5–15. PMID 18557129.
- ↑ 72.0 72.1 72.2 72.3 Ruffle JK (November 2014). "Molecular neurobiology of addiction: what's all the (Δ)FosB about?". Am. J. Drug Alcohol Abuse 40 (6): 428–437. doi:10.3109/00952990.2014.933840. PMID 25083822.
ΔFosB is an essential transcription factor implicated in the molecular and behavioral pathways of addiction following repeated drug exposure.
- ↑ 73.0 73.1 73.2 73.3 73.4 Nestler EJ (December 2013). "Cellular basis of memory for addiction". Dialogues Clin. Neurosci. 15 (4): 431–443. PMC 3898681. PMID 24459410.
- ↑ Robison AJ, Nestler EJ (November 2011). "Transcriptional and epigenetic mechanisms of addiction". Nat. Rev. Neurosci. 12 (11): 623–637. doi:10.1038/nrn3111. PMC 3272277. PMID 21989194.
ΔFosB serves as one of the master control proteins governing this structural plasticity.
- ↑ 75.0 75.1 75.2 75.3 75.4 75.5 75.6 75.7 75.8 75.9 75.10 75.11 75.12 75.13 75.14 75.15 75.16 75.17 75.18 75.19 75.20 75.21 75.22 Olsen CM (December 2011). "Natural rewards, neuroplasticity, and non-drug addictions". Neuropharmacology 61 (7): 1109–1122. doi:10.1016/j.neuropharm.2011.03.010. PMC 3139704. PMID 21459101.
- ↑ 76.0 76.1 76.2 76.3 76.4 Lynch WJ, Peterson AB, Sanchez V, Abel J, Smith MA (September 2013). "Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis". Neurosci. Biobehav. Rev. 37 (8): 1622–1644. doi:10.1016/j.neubiorev.2013.06.011. PMC 3788047. PMID 23806439.
.
- ↑ 77.0 77.1 77.2 77.3 Linke SE, Ussher M (January 2015). "Exercise-based treatments for substance use disorders: evidence, theory, and practicality". Am. J. Drug Alcohol Abuse 41 (1): 7–15. doi:10.3109/00952990.2014.976708. PMID 25397661.
- ↑ 78.0 78.1 Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and Addictive Disorders". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. p. 386. ISBN 9780071481274.
Currently, cognitive–behavioral therapies are the most successful treatment available for preventing the relapse of psychostimulant use.
- ↑ Greene SL, Kerr F, Braitberg G (October 2008). "Review article: amphetamines and related drugs of abuse". Emerg. Med. Australas 20 (5): 391–402. doi:10.1111/j.1742-6723.2008.01114.x. PMID 18973636.
- ↑ Albertson TE (2011). "Amphetamines". In Olson KR, Anderson IB, Benowitz NL, Blanc PD, Kearney TE, Kim-Katz SY, Wu AHB. Poisoning & Drug Overdose (6th ed.). New York: McGraw-Hill Medical. pp. 77–79. ISBN 9780071668330.
- ↑ "Glossary of Terms". Mount Sinai School of Medicine. Department of Neuroscience. Retrieved 9 February 2015.
- ↑ Kollins SH (May 2008). "A qualitative review of issues arising in the use of psycho-stimulant medications in patients with ADHD and co-morbid substance use disorders". Curr. Med. Res. Opin. 24 (5): 1345–1357. doi:10.1185/030079908X280707. PMID 18384709.
When oral formulations of psychostimulants are used at recommended doses and frequencies, they are unlikely to yield effects consistent with abuse potential in patients with ADHD.
- ↑ "Amphetamines: Drug Use and Abuse". Merck Manual Home Edition. Merck. February 2003. Archived from the original on 17 February 2007. Retrieved 28 February 2007.
- ↑ Perez-Mana C, Castells X, Torrens M, Capella D, Farre M (September 2013). Pérez-Mañá C, ed. "Efficacy of psychostimulant drugs for amphetamine abuse or dependence". Cochrane Database Syst. Rev. 9: CD009695. doi:10.1002/14651858.CD009695.pub2. PMID 23996457.
- ↑ Hyman SE, Malenka RC, Nestler EJ (July 2006). "Neural mechanisms of addiction: the role of reward-related learning and memory". Annu. Rev. Neurosci. 29: 565–598. doi:10.1146/annurev.neuro.29.051605.113009. PMID 16776597.
- ↑ 86.0 86.1 86.2 86.3 86.4 86.5 86.6 86.7 Robison AJ, Nestler EJ (November 2011). "Transcriptional and epigenetic mechanisms of addiction". Nat. Rev. Neurosci. 12 (11): 623–637. doi:10.1038/nrn3111. PMC 3272277. PMID 21989194.
- ↑ 87.0 87.1 87.2 87.3 87.4 Steiner H, Van Waes V (January 2013). "Addiction-related gene regulation: risks of exposure to cognitive enhancers vs. other psychostimulants". Prog. Neurobiol. 100: 60–80. doi:10.1016/j.pneurobio.2012.10.001. PMC 3525776. PMID 23085425.
- ↑ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 4: Signal Transduction in the Brain". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. p. 94. ISBN 9780071481274.
- ↑ Kanehisa Laboratories (29 October 2014). "Alcoholism – Homo sapiens (human)". KEGG Pathway. Retrieved 31 October 2014.
- ↑ Nestler EJ (January 2014). "Epigenetic mechanisms of drug addiction". Neuropharmacology. 76 Pt B: 259–268. doi:10.1016/j.neuropharm.2013.04.004. PMC 3766384. PMID 23643695.
- ↑ 91.0 91.1 Blum K, Werner T, Carnes S, Carnes P, Bowirrat A, Giordano J, Oscar-Berman M, Gold M (March 2012). "Sex, drugs, and rock 'n' roll: hypothesizing common mesolimbic activation as a function of reward gene polymorphisms". J. Psychoactive Drugs 44 (1): 38–55. doi:10.1080/02791072.2012.662112. PMC 4040958. PMID 22641964.
- ↑ 92.0 92.1 Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM (February 2013). "Natural and drug rewards act on common neural plasticity mechanisms with ΔFosB as a key mediator". J. Neurosci. 33 (8): 3434–3442. doi:10.1523/JNEUROSCI.4881-12.2013. PMC 3865508. PMID 23426671.
- ↑ Stoops WW, Rush CR (May 2014). "Combination pharmacotherapies for stimulant use disorder: a review of clinical findings and recommendations for future research". Expert Rev Clin Pharmacol 7 (3): 363–374. doi:10.1586/17512433.2014.909283. PMID 24716825.
Despite concerted efforts to identify a pharmacotherapy for managing stimulant use disorders, no widely effective medications have been approved.
- ↑ Perez-Mana C, Castells X, Torrens M, Capella D, Farre M (September 2013). "Efficacy of psychostimulant drugs for amphetamine abuse or dependence". Cochrane Database Syst. Rev. 9: CD009695. doi:10.1002/14651858.CD009695.pub2. PMID 23996457.
To date, no pharmacological treatment has been approved for [addiction], and psychotherapy remains the mainstay of treatment. ... Results of this review do not support the use of psychostimulant medications at the tested doses as a replacement therapy
- ↑ Forray A, Sofuoglu M (February 2014). "Future pharmacological treatments for substance use disorders". Br. J. Clin. Pharmacol. 77 (2): 382–400. doi:10.1111/j.1365-2125.2012.04474.x. PMC 4014020. PMID 23039267.
- ↑ 96.0 96.1 Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 5: Excitatory and Inhibitory Amino Acids". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. pp. 124–125. ISBN 9780071481274.
- ↑ Srisurapanont M, Jarusuraisin N, Kittirattanapaiboon P (October 2001). Srisurapanont M, ed. "Treatment for amphetamine dependence and abuse". Cochrane Database Syst. Rev. (4): CD003022. doi:10.1002/14651858.CD003022. PMID 11687171.
Although there are a variety of amphetamines and amphetamine derivatives, the word "amphetamines" in this review stands for amphetamine, dextroamphetamine and methamphetamine only.
- ↑ 98.0 98.1 98.2 98.3 Shoptaw SJ, Kao U, Heinzerling K, Ling W (April 2009). Shoptaw SJ, ed. "Treatment for amphetamine withdrawal". Cochrane Database Syst. Rev. (2): CD003021. doi:10.1002/14651858.CD003021.pub2. PMID 19370579.
- ↑ "Adderall IR Prescribing Information" (PDF). United States Food and Drug Administration. Barr Laboratories, Inc. March 2007. Retrieved 4 November 2013.
- ↑ "Dexedrine Medication Guide" (PDF). United States Food and Drug Administration. Amedra Pharmaceuticals LLC. May 2013. Retrieved 4 November 2013.
- ↑ "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. Retrieved 30 December 2013.
- ↑ Advokat C (July 2007). "Update on amphetamine neurotoxicity and its relevance to the treatment of ADHD". J. Atten. Disord. 11 (1): 8–16. doi:10.1177/1087054706295605. PMID 17606768.
- ↑ "Amphetamine". Hazardous Substances Data Bank. National Library of Medicine. Retrieved 26 February 2014.
Direct toxic damage to vessels seems unlikely because of the dilution that occurs before the drug reaches the cerebral circulation.
- ↑ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 15: Reinforcement and addictive disorders". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. p. 370. ISBN 9780071481274.
Unlike cocaine and amphetamine, methamphetamine is directly toxic to midbrain dopamine neurons.
- ↑ Sulzer D, Zecca L (February 2000). "Intraneuronal dopamine-quinone synthesis: a review". Neurotox. Res. 1 (3): 181–195. doi:10.1007/BF03033289. PMID 12835101.
- ↑ Miyazaki I, Asanuma M (June 2008). "Dopaminergic neuron-specific oxidative stress caused by dopamine itself". Acta Med. Okayama 62 (3): 141–150. PMID 18596830.
- ↑ Hofmann FG (1983). A Handbook on Drug and Alcohol Abuse: The Biomedical Aspects (2nd ed.). New York, USA: Oxford University Press. p. 329. ISBN 9780195030570.
- ↑ 108.0 108.1 108.2 108.3 108.4 108.5 "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. December 2013. pp. 8–10. Retrieved 30 December 2013.
- ↑ 109.0 109.1 109.2 Krueger SK, Williams DE (June 2005). "Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism". Pharmacol. Ther. 106 (3): 357–387. doi:10.1016/j.pharmthera.2005.01.001. PMC 1828602. PMID 15922018.
- ↑ "Amphetamine: Biomolecular Interactions and Pathways". PubChem Compound. National Center for Biotechnology Information. Retrieved 13 October 2013.
- ↑ 111.0 111.1 111.2 111.3 Lewin AH, Miller GM, Gilmour B (December 2011). "Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class". Bioorg. Med. Chem. 19 (23): 7044–7048. doi:10.1016/j.bmc.2011.10.007. PMC 3236098. PMID 22037049.
- ↑ Smith R C, Davis J M (June 1977). "Comparative effects of d-amphetamine, l-amphetamine, and methylphenidate on mood in man". Psychopharmacology 53 (1): 1–12. doi:10.1007/bf00426687. PMID 407607.
- ↑ Glaser PE, Thomas TC, Joyce BM, Castellanos FX, Gerhardt GA (March 2005). "Differential effects of amphetamine isomers on dopamine release in the rat striatum and nucleus accumbens core". Psychopharmacology (Berl.) 178 (2–3): 250–8. doi:10.1007/s00213-004-2012-6. PMID 15719230.
- ↑ "better clinical response to levoamphetamine".
- ↑ Arnold LE (2000). "Methyiphenidate vs. Amphetamine: Comparative review". Journal of Attention Disorders 3 (4): 200–11. doi:10.1177/108705470000300403.
- ↑ "Pharmacology". Dextroamphetamine. DrugBank. University of Alberta. 8 February 2013. Retrieved 5 November 2013.
- ↑ 117.0 117.1 117.2 117.3 117.4 117.5 117.6 117.7 117.8 117.9 117.10 117.11 117.12 117.13 117.14 "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. pp. 12–13. Retrieved 30 December 2013.
- ↑ "Pharmacology". Amphetamine. DrugBank. University of Alberta. 8 February 2013. Retrieved 5 November 2013.
- ↑ 119.0 119.1 119.2 119.3 "Pharmacology and Biochemistry". Amphetamine. Pubchem Compound. National Center for Biotechnology Information. Retrieved 12 October 2013.
- ↑ "Biological Half-Life". AMPHETAMINE. United States National Library of Medicine – Toxnet. Hazardous Substances Data Bank. Retrieved 5 January 2014.
- ↑ Richard RA (1999). "Route of Administration". Chapter 5—Medical Aspects of Stimulant Use Disorders. National Center for Biotechnology Information Bookshelf. Treatment Improvement Protocol 33. Substance Abuse and Mental Health Services Administration.
- ↑ Lemke TL, Williams DA, Roche VF, Zito W (2013). Foye's Principles of Medicinal Chemistry (7th ed.). Philadelphia, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 648. ISBN 9781609133450.
Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.
- ↑ Taylor KB (January 1974). "Dopamine-beta-hydroxylase. Stereochemical course of the reaction" (PDF). J. Biol. Chem. 249 (2): 454–458. PMID 4809526. Retrieved 6 November 2014.
Dopamine-β-hydroxylase catalyzed the removal of the pro-R hydrogen atom and the production of 1-norephedrine, (2S,1R)-2-amino-1-hydroxyl-1-phenylpropane, from d-amphetamine.
- ↑ Horwitz D, Alexander RW, Lovenberg W, Keiser HR (May 1973). "Human serum dopamine-β-hydroxylase. Relationship to hypertension and sympathetic activity". Circ. Res. 32 (5): 594–599. doi:10.1161/01.RES.32.5.594. PMID 4713201.
Subjects with exceptionally low levels of serum dopamine-β-hydroxylase activity showed normal cardiovascular function and normal β-hydroxylation of an administered synthetic substrate, hydroxyamphetamine.
- ↑ Cashman JR, Xiong YN, Xu L, Janowsky A (March 1999). "N-oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): role in bioactivation and detoxication". J. Pharmacol. Exp. Ther. 288 (3): 1251–1260. PMID 10027866.
- ↑ 126.0 126.1 "Substrate/Product". butyrate-CoA ligase. BRENDA. Technische Universität Braunschweig. Retrieved 7 May 2014.
- ↑
- ↑ 128.0 128.1 128.2 Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G (September 2002). "Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection". J. Pharm. Biomed. Anal. 30 (2): 247–255. doi:10.1016/S0731-7085(02)00330-8. PMID 12191709.
- ↑ "Compound Summary". p-Hydroxyamphetamine. PubChem Compound. National Center for Biotechnology Information. Retrieved 15 October 2013.
- ↑ "Compound Summary". p-Hydroxynorephedrine. PubChem Compound. National Center for Biotechnology Information. Retrieved 15 October 2013.
- ↑ "Compound Summary". Phenylpropanolamine. PubChem Compound. National Center for Biotechnology Information. Retrieved 15 October 2013.
- ↑ 132.0 132.1 132.2 132.3 132.4 Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends Pharmacol. Sci. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- ↑ "APPROVAL LETTER" (PDF). United States Food and Drug Administration. Retrieved 30 December 2013.
- ↑ "August 2006 News Archives: Barr and Shire Sign Three Agreements". GenericsWeb. Retrieved 30 December 2013.
WOODCLIFF LAKE, N.J., Aug. 14 /PRNewswire-FirstCall/ – Barr Pharmaceuticals, Inc. today announced that its subsidiary Duramed Pharmaceuticals, Inc. and Shire plc have signed a Product Acquisition Agreement for ADDERALL(R) (immediate-release mixed amphetamine salts) tablets and a Product Development Agreement for six proprietary products, and that its subsidiary Barr Laboratories, Inc. (Barr) has signed a Settlement and License Agreement relating to the resolution of two pending patent cases involving Shire's ADDERALL XR(R) ...
- ↑ "Teva Completes Acquisition of Barr". Drugs.com. Retrieved 31 October 2011.
- ↑ "Teva sells 1st generic of Adderall XL in US". Forbes Magazine. Associated Press. 2 April 2009. Archived from the original on 9 April 2009. Retrieved 22 April 2009.
- ↑ "Dexedrine Medication Guide" (PDF). United States Food and Drug Administration. May 2013. Retrieved 4 November 2013.
- ↑ "REGULATORY NEWS: Richwood's Adderall". Health News Daily. 22 February 1996. Retrieved 29 May 2013.
- ↑ The Minister and Attorney General. "Controlled Drugs and Substances Act". Justice Laws Website. Government of Canada.
- ↑ "Importing or Bringing Medication into Japan for Personal Use". Japan Ministry of Health, Labour and Welfare.
- ↑ "Thailand Law" (PDF). Government of Thailand. Retrieved 23 May 2013.
- ↑ "Class A, B and C drugs". Home Office, Government of the United Kingdom. Archived from the original on 4 August 2007. Retrieved 23 July 2007.
- ↑ Substance Abuse and Mental Health Services Administration. "Trends in Methamphetamine/Amphetamine Admissions to Treatment: 1993–2003". The Drug and Alcohol Services Information System (DASIS) Report. United States Department of Health and Human Services. Retrieved 28 February 2007.
- ↑ United Nations Office on Drugs and Crime (2007). Preventing Amphetamine-type Stimulant Use Among Young People: A Policy and Programming Guide (PDF). New York: United Nations. ISBN 92-1-148223-2.
- ↑ International Narcotics Control Board. "List of psychotropic substances under international control" (PDF). Vienna: United Nations. Archived (PDF) from the original on 5 December 2005. Retrieved 19 November 2005.
| ||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||