Acridine
 | |
| Names | |
|---|---|
| IUPAC name
Acridine | |
| Systematic IUPAC name
Dibenzo[b,e]pyridine[1] | |
| Other names
2,3-Benzoquinoline[2] | |
| Identifiers | |
| 260-94-6 | |
| ChEBI | CHEBI:36420 |
| ChEMBL | ChEMBL39677 |
| ChemSpider | 8860 |
| EC number | 205-971-6 |
| |
| Jmol-3D images | Image Image |
| PubChem | 9215 |
| RTECS number | AR7175000 |
| |
| Properties | |
| Molecular formula |
C13H9N |
| Molar mass | 179.22 g·mol−1 |
| Appearance | White powder |
| Odor | Irritating |
| Density | 1.005 g/cm3 (20 °C)[1] |
| Melting point | 106–110 °C (223–230 °F; 379–383 K) at 760 mmHg[1] |
| Boiling point | 344.86 °C (652.75 °F; 618.01 K) at 760 mmHg[1] |
| 46.5 mg/L[1] | |
| Solubility | Soluble in CCl4, alcohols, (C2H5)2O, C6H6[1] |
| log P | 3.4[1] |
| Vapor pressure | 0.34 kPa (150 °C) 2.39 kPa (200 °C) 11.13 kPa (250 °C)[3] |
| Acidity (pKa) | 5.58 (20 °C)[1] |
| UV-vis (λmax) | 392 nm[4] |
| Thermochemistry | |
| Specific heat capacity (C) |
205.07 J/mol·K[3] |
| Std molar entropy (S |
208.03 J/mol·K[3] |
| Std enthalpy of formation (ΔfH |
179.4 kJ/mol[1] |
| Std enthalpy of combustion (ΔcH |
6581.3 kJ/mol[3] |
| Hazards | |
| GHS pictograms |  [4] [4] |
| GHS signal word | Danger |
| H302[4] | |
| EU classification | |
| R-phrases | R22, R36/37/38 |
| S-phrases | (S2), S24/25, S36 |
| NFPA 704 | |
| LD50 (Median lethal dose) |
500 mg/kg (mice, oral)[2] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 0.2 mg/m3 (benzene-soluble fraction)[5] |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Acridine is an organic compound and a nitrogen heterocycle with the formula C13H9N. Acridines are substituted derivatives of the parent ring. It is a planar molecule that is structurally related to anthracene with one of the central CH groups is replaced by nitrogen. Like the related molecule pyridine and quinoline, acridine is mildly basic. It is an almost colorless solid. There are no commercial applications of acridines but at one time acridine dyes were popular. It crystallizes in needles.
Isolation and syntheses
Carl Gräbe and Heinrich Caro first isolated acridine in 1870 from coal tar. Acridine is separated from coal tar by extracting with dilute sulfuric acid. Addition of potassium dichromate to this solution precipitates acridine bichromate. The bichromate is decomposed using ammonia.
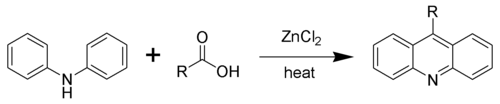
Acridine and its derivatives can be prepared by many synthetic processes. In the Bernthsen acridine synthesis, diphenylamine is condenses with carboxylic acids in the presence of zinc chloride. When formic acid as the carboxylic acid, the reaction yields the parent acridine. With the higher larger carboxylic acids, the derivatives substituted at the meso carbon atom are generated.
Other older methods for the organic synthesis of acridines include condensing diphenylamine with chloroform in the presence of aluminium chloride, by passing the vapours of orthoaminodiphenylmethane over heated litharge, by heating salicylaldehyde with aniline and zinc chloride or by distilling acridone (9-position a carbonyl group) over zinc dust.[6] Another classic method for the synthesis of acridones is the Lehmstedt-Tanasescu reaction.
Reactions
Acridine displays the reactions expected of an unsaturated N-heterocycle. It undergoes N-alkylation with alkyl iodides to form alkyl acridinium iodides, which are readily transformed by the action of alkaline potassium ferricyanide to N-alkyl acridones.
Basicity
Acridine and its homologues are weakly basic. Acridine is a photobase which have a ground state pKa of 5.1, which is similar to that of pyridine, and have an excited state pKa of 10.6.[7] It also shares properties with quinoline.
Reduction and oxidation
Acridanes can be reduced to the 9,10-dihydroacridines, sometimes called leuco acridines. Reaction with potassium cyanide gives the 9-cyano-9,10-dehydro derivative. On oxidation with potassium permanganate, it yields acridinic acid (C9H5N(CO2H)2) otherwise known as quinoline-1,2-dicarboxylic acid.[6] Acridine is easily oxidized by peroxymonosulfuric acid to the acridine amine oxide. The carbon 9-position of acridine is activated for addition reactions.[8]
Applications
Several dyes and drugs feature the acridine skeleton.[9] Many acridines, such as proflavine, also have antiseptic properties. Acridine and related derivatives (such as amsacrine) bind to DNA and RNA due to their abilities to intercalate. Acridine orange (3,6-dimethylaminoacridine) is a nucleic acid-selective metachromatic stain useful for cell cycle determination.
Dyes
At one time acridine dyes were commercially significant, but they are now uncommon because they are not lightfast. Acridine dyes are prepared by condensation of 1,3-diaminobenzene derivatives. Illustrative is the reaction of 2,4-diaminotoluene with acetaldehyde:[10]
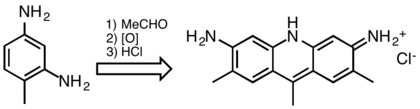
9-Phenylacridine is the parent base of chrysaniline or 3,6-diamino-9-phenylacridine, which is the chief constituent of the dyestuff phosphine (not to be confused with phosphine gas), a by-product in the manufacture of rosaniline. Chrysaniline forms red-coloured salts, which dye silk and wool a fine yellow; and the solutions of the salts are characterized by their fine yellowish-green fluorescence. Chrysaniline was synthesized by O. Fischer and G. Koerner by condensing ortho-nitrobenzaldehyde with aniline, the resulting ortho-nitro-para-diamino-triphenylmethane being reduced to the corresponding orthoamino compound, which on oxidation yields chrysaniline. Benzoflavin, an isomer of chrysaniline, is also a dye-stuff, and has been prepared by K. Oehler from meta-phenylenediamine and benzaldehyde. These substances condense to form tetra-aminotriphenylmethane, which, on heating with acids, loses ammonia and yields 3,6-diamino-9,10-dihydrophenylacridine, from which benzoflavin is obtained by oxidation. It is a yellow powder, soluble in hot water.[6]
Safety
Acridine is a skin irritant. Its LD50 (rats, oral) is 2000 mg/kg and 500 mg/kg (mice, oral).[2]
See also
Acridarsine is formally derived from acridine by replacing the nitrogen atom with one of arsenic, and acridophosphine by replacing it with one of phosphorus.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ↑ 2.0 2.1 2.2
- ↑ 3.0 3.1 3.2 3.3 Acridine in Linstrom, P.J.; Mallard, W.G. (eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69. National Institute of Standards and Technology, Gaithersburg MD. http://webbook.nist.gov (retrieved 2014-06-22)
- ↑ 4.0 4.1 4.2 Sigma-Aldrich Co., Acridine. Retrieved on 2014-06-22.
- ↑ "NIOSH Pocket Guide to Chemical Hazards #0145". National Institute for Occupational Safety and Health (NIOSH).
- ↑ 6.0 6.1 6.2
 One or more of the preceding sentences incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). "Acridine". Encyclopædia Britannica 1 (11th ed.). Cambridge University Press. p. 155.
One or more of the preceding sentences incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). "Acridine". Encyclopædia Britannica 1 (11th ed.). Cambridge University Press. p. 155. - ↑ Joseph R. Lakowicz. Principles of Fluorescence Spectroscopy 3rd edition. Springer (2006). ISBN 978-0387-31278-1. Chapter 7. page 260.
- ↑ G. Collin, H. Höke,"Acridine" in Ullmann's Encyclopedia of Industrial Chemistry 2012, Wiley-VCH, Weinheim.doi:10.1002/14356007.a01_147
- ↑ Denny, W. A., "Acridine derivatives as chemotherapeutic agents", Curr. Med. Chem. 2002, volume 9, 1655. doi:10.2174/0929867023369277
- ↑ Thomas Gessner and Udo Mayer "Triarylmethane and Diarylmethane Dyes" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim.doi:10.1002/14356007.a27_179
- Synthesis of Acridine-based DNA Bis-intercalating Agents Gerard P. Moloney, David P. Kelly, P. Mack Molecules 2001, 6, 230-243 open source
External links
| Wikimedia Commons has media related to Acridine. |
| Wikisource has original text related to this article: |
- Synthesis of acridone in Organic Syntheses 19:6; Coll. Vol. 2:15 from o-chlorobenzoic acid and aniline in a Goldberg reaction.
- Synthesis of 9-aminoacridine in Organic Syntheses 22:5; Coll. Vol. 3:53. from N-phenylanthranilic acid.