Acetochlor
 | |
| Names | |
|---|---|
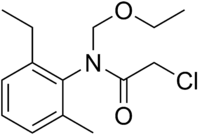
| IUPAC name
2-Chloro-N-(ethoxymethyl)-N-(2-ethyl-6-methylphenyl)acetamide | |
| Other names
Azetochlor | |
| Identifiers | |
| 34256-82-1 | |
| ChEBI | CHEBI:2394 |
| ChEMBL | ChEMBL1517425 |
| ChemSpider | 1911 |
| |
| Jmol-3D images | Image |
| KEGG | C10925 |
| PubChem | 1988 |
| |
| UNII | 8L08WMO94K |
| Properties | |
| C14H20ClNO2 | |
| Molar mass | 269.767 g/mol |
| Density | 1.100 at 30 °C 1.136 at 20 °C |
| Melting point | <0 °C |
| 223 ppm | |
| Hazards | |
| Flash point | >100 °C |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Acetochlor is a herbicide developed by Monsanto Company and Zeneca. It is a member of the class of herbicides known as chloroacetanilides. Its mode of action is elongase inhibition, and inhibition of geranylgeranyl pyrophosphate (GGPP) cyclisation enzymes, part of the gibberellin pathway. It carries high risks of environmental contamination.[2]
Uses
In the US Acetochlor was registered by the EPA as a direct substitute for many herbicides of known concern. The EPA imposed several restrictions and conditions on the use of acetochlor.[3]
It is homologuated for pre-emergence application or for pre-planting application with soil incorporation, in corn. (maize) at 5 litres / hectare (1835g / hectare of a.i.)[4] It's the main active ingredient in Acenit, Keystone, Guardian, Harness, Relay, Sacemid, Surpass, Top-Hand, Trophy and Winner.
It is used to control weeds in corn, and is particularly useful as a replacement for atrazine in the case of some important weeds.
Safety
Acetochlor has been classified as a probable human carcinogen.[1][3]Acetochlor, as Alachlor, can cause nasal turbinate tumors via the generation of a common tissue reactive metabolite that leads to cytotoxicity and regenerative proliferation in the nasal epithelium.[5]
It's also a well-established thyroid disruptor[6]
Human health effects from acetochlor at low environmental doses or at biomonitored levels from low environmental exposures are unknown.[7]
Ecologic effects
In the US Acetochlor is the third most frequently detected herbicide in natural waters[8]
Acetochlor can accelerate metamorphosis in amphibians.[9] It can also affect the development of fish.[10]
See also
References
- ↑ 1.0 1.1 Cornell University Extension Toxicology Network Pesticide Information Profile on Acetochlor
- ↑ Arregui, M.; Sánchez, D.; Althaus, R.; Scotta, R.; Bertolaccini, I. (2010). "Assessing the risk of pesticide environmental impact in several Argentinian cropping systems with a fuzzy expert indicator". Pest management science 66 (7): 736–740. doi:10.1002/ps.1935. PMID 20232283.
- ↑ 3.0 3.1 "Acetochlor". EPA. Retrieved 2 April 2010.
- ↑ e-phy
- ↑ "Cumulative Risk Assessment for the Chloroacetanilides" (PDF). EPA. 2006-03-29. Retrieved 2 April 2010.
- ↑ Turque, N.; Palmier, K.; Le Mével, S.; Alliot, C.; Demeneix, B. A. (2005). "A Rapid, Physiologic Protocol for Testing Transcriptional Effects of Thyroid-Disrupting Agents in Premetamorphic Xenopus Tadpoles". Environmental health perspectives 113 (11): 1588–1593. doi:10.1289/ehp.7992. PMC 1310923. PMID 16263516.
- ↑ "National Report on Human Exposure to Environmental Chemicals". CDC. 11 February 2010. Retrieved 2 April 2010.
- ↑ Foley, M.; Sigler, V.; Gruden, C. (2008). "A multiphasic characterization of the impact of the herbicide acetochlor on freshwater bacterial communities.". The ISME journal 2 (1): 56–66. doi:10.1038/ismej.2007.99. PMID 18180747.
- ↑ Li, W.; Zha, J.; Li, Z.; Yang, L.; Wang, Z. (2009). "Effects of exposure to acetochlor on the expression of thyroid hormone related genes in larval and adult rare minnow (Gobiocypris rarus)". Aquatic Toxicology 94 (2): 87–93. doi:10.1016/j.aquatox.2009.06.002. PMID 19577311.
- ↑ Li, W.; Zha, J.; Li, Z.; Yang, L.; Wang, Z. (2009). "Effects of exposure to acetochlor on the expression of thyroid hormone related genes in larval and adult rare minnow (Gobiocypris rarus)". Aquatic Toxicology 94 (2): 87–93. doi:10.1016/j.aquatox.2009.06.002. PMID 19577311.
External links
| ||||||||||||||||||||||||||||||||||||||||||