Acentric factor
The acentric factor 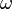 is a conceptual number introduced by Kenneth Pitzer in 1955, proven to be very useful in the description of matter.[1] It has become a standard for the phase characterization of single & pure components. The other state description parameters are molecular weight, critical temperature, critical pressure, and critical volume.The acentric factor is said to be a measure of the non-sphericity (centricity) of molecules.[2]
is a conceptual number introduced by Kenneth Pitzer in 1955, proven to be very useful in the description of matter.[1] It has become a standard for the phase characterization of single & pure components. The other state description parameters are molecular weight, critical temperature, critical pressure, and critical volume.The acentric factor is said to be a measure of the non-sphericity (centricity) of molecules.[2]
It is defined as:
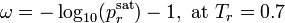 .
.
where
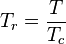 is the reduced temperature,
is the reduced temperature,
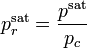 is the reduced pressure saturation of vapors.
is the reduced pressure saturation of vapors.
For many monatomic fluids
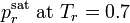 ,
,
is close to 0.1, therefore 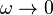 . In many cases,
. In many cases, 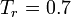 lies above the boiling temperature of gases at atmosphere pressure.
lies above the boiling temperature of gases at atmosphere pressure.
Values of 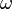 can be determined for any fluid from
can be determined for any fluid from 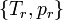 , and a vapor measurement from
, and a vapor measurement from 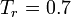 , and for many liquid state matter is tabulated into many thermodynamical tables.
, and for many liquid state matter is tabulated into many thermodynamical tables.
The definition of 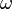 gives zero-value for the noble gases argon, krypton, and xenon.
gives zero-value for the noble gases argon, krypton, and xenon. 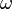 is almost exactly zero for other spherical molecules.[2]
Experimental data yields compressibility factors for all fluids that are correlated by the same curves when
is almost exactly zero for other spherical molecules.[2]
Experimental data yields compressibility factors for all fluids that are correlated by the same curves when 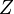 (compressibility factor) is represented as a function of
(compressibility factor) is represented as a function of 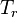 and
and 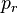 . This is the basis premises of three-parameter theorem of corresponding states:
. This is the basis premises of three-parameter theorem of corresponding states:
All fluids at any 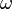 -value, in
-value, in 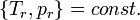 conditions, have about the same
conditions, have about the same 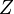 -value, and same degree of convergence.
-value, and same degree of convergence.
Values of some common gases
| Molecule | Acentric Factor[3] |
| Acetylene | 0.187 |
| Ammonia | 0.253 |
| Argon | 0.000 |
| Carbon Dioxide | 0.228 |
| Decane | 0.484 |
| Helium | -0.390 |
| Hydrogen | -0.220 |
| Krypton | 0.000 |
| Neon | 0.000 |
| Nitrogen | 0.040 |
| Nitrous Oxide | 0.142 |
| Oxygen | 0.022 |
| Xenon | 0.000 |
See also
- Equation of state
- Reduced pressure
- Reduced temperature
References
- ↑ Adewumi, Michael. "Acentric Factor and Corresponding States". Pennsylvania State University. Retrieved 2013-11-06.
- ↑ 2.0 2.1 Saville, G. (2006). "ACENTRIC FACTOR". A-to-Z Guide to Thermodynamics, Heat and Mass Transfer, and Fluids Engineering. doi:10.1615/AtoZ.a.acentric_factor.
- ↑ Yaws, Carl L. (2001). Matheson Gas Data Book. McGraw-Hill.