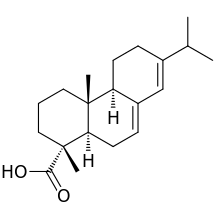
Abietic acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Abieta-7,13-dien-18-oic acid | |
| Other names
(1R,4aR,4bR,10aR)-7-Isopropyl-1,4a-dimethyl-1,2,3,4,4a,4b,5,6,10,10a-decahydrophenanthrene-1-carboxylic acid; Abietinic acid; Sylvic acid | |
| Identifiers | |
| 514-10-3 | |
| ChEBI | CHEBI:28987 |
| ChEMBL | ChEMBL71893 |
| ChemSpider | 10127 |
| EC number | 208-173-3 |
| |
| Jmol-3D images | Image |
| KEGG | C06087 |
| PubChem | 10569 |
| RTECS number | TP8580000 |
| |
| UNII | V3DHX33184 |
| Properties | |
| Molecular formula |
C20H30O2 |
| Molar mass | 302.45 g·mol−1 |
| Appearance | Yellow resinous powder, crystals or chunks. Monoclinic plates (from EtOH/water). Colorless solid when pure. |
| Density | 1.06 g/mL |
| Melting point | 172–175 °C (342–347 °F; 445–448 K) [1] |
| Insoluble[1] | |
| Solubility in other solvents | Very soluble in acetone, petroleum ether, Et2O, and ethanol |
| Hazards | |
| MSDS | MSDS |
| Main hazards | Irritant |
| R-phrases | R36/37/38 |
| S-phrases | S26 S36 |
| NFPA 704 | |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
Abietic acid (also known as abietinic acid or sylvic acid) is an organic compound that occurs widely in trees. It is the primary component of resin acid, is the primary irritant in pine wood and resin, isolated from rosin (via isomerization) and is the most abundant of several closely related organic acids that constitute most of rosin, the solid portion of the oleoresin of coniferous trees. Its ester is called an abietate.[2]
Preparation
Abietic acid is extracted from tree rosin.[3] The pure material is a colorless solid, but commercial samples are usually a glassy or partly crystalline yellowish solid that melts at temperatures as low as 85 °C (185 °F).[4]
It belongs to the abietane diterpene group of organic compounds derived from four isoprene units. It is used in lacquers, varnishes, and soaps, and for the analysis of resins and the preparation of metal resinates. It is found in Pinus insularis (Khasi Pine), Pinus kesiya Royle, Pinus strobus (Eastern White Pine), and Pinus sylvestris (Scots Pine).[5]
Uses
Rosin has been used for centuries for caulking ships.[4] It is also rubbed on the bows of musical instruments to make them less slippery.[4] In modern times methods have been developed for improving the properties of the rosin acids, which are otherwise soft, tacky, and low-melting and subject to rapid deterioration by oxidation in air. Stability is greatly increased by heat treatment.
Resin acids are converted into ester gum by reaction with controlled amounts of glycerol or other polyhydric alcohols. Ester gum has drying properties and is used in paints, varnishes, and lacquers.[4]
Rosin has been used to depackage integrated circuits from their epoxy coatings.[6]
In vitro effects
The 50% ethanol extracts from Resina pini of Pinus sp. (Pinaceae) showed inhibitory activity against testosterone 5α-reductase prepared from rat prostate. The fraction responsible for this activity was purified, and the active constituent was isolated and identified as abietic acid which exhibited potent testosterone 5α-reductase inhibitory activity in vitro.[7]
Safety
Abietic acid is considered a "nonhazardous natural substances" in tall oil.[2] In the U.S., it is listed in the Toxic Substances Control Act inventory. Abietic acid is a contact allergen;[8] however, compounds resulting from its oxidation by air elicit stronger responses.[9] It is soluble in alcohols, acetone, and ethers.
References
- ↑ 1.0 1.1 Merck Index, 12th Edition, 3. Abietic Acid
- ↑ 2.0 2.1 Lars-Hugo Norlin "Tall Oil" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim.doi:10.1002/14356007.a26_057
- ↑ G. C. Harris and T. F. Sanderson (1963). "Abietic Acid". Org. Synth. 32: 1.; Coll. Vol. 4, p. 1
- ↑ 4.0 4.1 4.2 4.3 Hoiberg, Dale H., ed. (2010). "abietic acid". Encyclopedia Britannica. I: A-ak Bayes (15th ed.). Chicago, IL: Encyclopedia Britannica Inc. p. 32. ISBN 978-1-59339-837-8.
- ↑ "Abietic Acid". Dr. Duke's Phytochemical and Ethnobotanical Databases. Retrieved 13 January 2012.
- ↑ "Deencapsulation of ICs using heated rosin".
- ↑ Seong-Soo Roh, Moon-Ki Park and Yong-ung Kim (2010). "Abietic Acid from Resina Pini of Pinus Species as a Testosterone 5α-Reductase Inhibitor". J. Health Sci. 56 (4): 451–455. doi:10.1248/jhs.56.451.
- ↑ El Sayed, F; Manzur, F; Bayle, P; Marguery, MS; Bazex, J (1995). "Contact urticaria from abietic acid". Contact dermatitis 32 (6): 361–2. doi:10.1111/j.1600-0536.1995.tb00628.x. PMID 7554886.
- ↑ Hausen, BM; Krohn, K; Budianto, E (1990). "Contact allergy due to colophony (VII). Sensitizing studies with oxidation products of abietic and related acids". Contact dermatitis 23 (5): 352–8. doi:10.1111/j.1600-0536.1990.tb05171.x. PMID 2096024.
