8-Prenylnaringenin
 | |
| Names | |
|---|---|
| IUPAC name
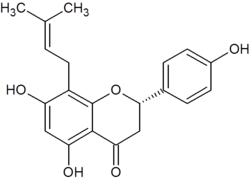
(2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-8-(3-methylbut-2-enyl)-2,3-dihydrochromen-4-one | |
| Other names
Hopein | |
| Identifiers | |
| ChEBI | CHEBI:50207 |
| ChemSpider | 421848 |
| |
| Jmol-3D images | Image |
| PubChem | 480764 |
| |
| Properties | |
| C20H20O5 | |
| Molar mass | 340.36 g/mol |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
8-Prenylnaringenin known as flavaprenin, (S)-8-dimethylallylnaringenin, hopein or 8-PN is a prenylflavonoid. It is the most estrogenic phytoestrogen known.[1] Its effects are similar to, but weaker than estradiol.[2]
8-Prenylnaringenin is found in hops (Humulus lupulus).[3] It can be produced from isoxanthohumol in fungal cells cultures,[4] and by flora in the human intestine.[1][5]
Properties
In an in vitro study, 8-Prenylnaringenin and its synthesized derivatives of it demonstrated to have anticancer activity.[6] This prenylflavonoid was shown to preserve bone density.[1]
Estrogenic
8-Prenylnaringenin has been demonstrated to reduce hot flashes.[1][7] 8-Prenylnaringenin also influences prolactin, and increases other estrogenic responses.[8] 8-prenylnaringenin binds to and activates ER-α more times than it does to ER-β.[1][2][9] This prenylflavonoid also interacts with the progesterone receptor.[8]
This prenylflavanoid has drawn interest in the study of hormone replacement therapy, and it is comparable to selective estrogen-receptor modulators.[10][11]
In an in vivo study, 8-prenylnaringenin has activated proliferation of mammary cells.[8] At the concentration found in beer, it is unlikely to have an estrogenic effect in breast tissue.[12] Prenylflavonoids from hops, namely 8-prenylnaringenin, are common in herbal breast enlargement preparations.[13]
Luteinizing hormone (LH) and follicle stimulating hormone (FSH) are reduced by 8-prenylnaringenin.[8] 8-Prenylnaringenin adversely affects male sperm.[14] The role 8-prenylnaringenin plays in fertility requires further research.
Chemistry
The enzyme naringenin 8-dimethylallyltransferase uses dimethylallyl diphosphate and (−)-(2S)-naringenin to produce diphosphate and sophoraflavanone B (8-Prenylnaringenin).
The enzyme 8-dimethylallylnaringenin 2'-hydroxylase uses sophoraflavanone B (8-prenylnaringenin), NADPH, H+ and O2 to produce leachianone G, NADP+ and H2O.
Synthesized derivatives of 8-prenylnaringenin are: 7,4′-di-O-methyl-8-prenylnaringenin; 7-O-pentyl-8-prenylnaringenin; 7,4′-Di-O-allyl-8-prenylnaringenin; 7,4′-Di-O-acetyl-8-prenylnaringenin; and 7,4′-Di-O-palmitoyl-8-prenylnaringenin.[6]
8-neopentylnaringenin and 8-n-heptylnaringenin are synthetic forms of 8-Prenylnaringenin.[15]
Etymology
There is another compound, 8-isopentenylnaringenin,[1] also known as sophoraflavanone B, from sophora flavescens, that could properly be called 8-prenylnaringenin by scientific naming convention.[16]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Keiler; Zierau; Kretzschmar (2013). "Hop Extracts and Hop Substances in Treatment of Menopausal Complaints". Planta Medica 2013; 79(07): 576-579 79 (7): 576–567. doi:10.1055/s-0032-1328330. PMID 23512496.
- ↑ 2.0 2.1 Hajirahimkhan; Dietz; Bolton (2013). "Botanical Modulation of Menopausal Symptoms: Mechanisms of Action?". Planta Med 79 (7): 538–553. doi:10.1055/s-0032-1328187. PMC 3800090. PMID 23408273.
- ↑ Nikolic, D; Li, Y; Chadwick, LR; Grubjesic, S; Schwab, P; Metz, P; Van Breemen, RB (2004). "Metabolism of 8-prenylnaringenin, a potent phytoestrogen from hops (Humulus lupulus), by human liver microsomes". Drug metabolism and disposition: the biological fate of chemicals 32 (2): 272–9. doi:10.1124/dmd.32.2.272. PMID 14744951.
- ↑ Production of 8-Prenylnaringenin from Isoxanthohumol through Biotransformation by Fungi Cells. Ming-liang Fu, Wei Wang, Feng Chen, Ya-chen Dong, Xiao-jie Liu, Hui Ni and Qi-he Chen, J. Agric. Food Chem., 2011, volume 59, issue 13, pages 7419–7426, doi:10.1021/jf2011722
- ↑ Possemiers, S. et al. (July 2006). "The Prenylflavonoid Isoxanthohumol from Hops (Humulus lupulus L.) Is Activated into the Potent Phytoestrogen 8-Prenylnaringenin In Vitro and in the Human Intestine". Journal of Nutrition (American Society for Nutrition) 136 (7): 1862–1867. PMID 16772450.
- ↑ 6.0 6.1 Anioł, Mirosław (January 7, 2012). "Antiproliferative activity and synthesis of 8-prenylnaringenin derivatives by demethylation of 7-O- and 4′-O-substituted isoxanthohumols". Med Chem Res. doi:10.1007/s00044-011-9967-8. Retrieved 2013-04-07.
- ↑ Bowe, James (November 15, 2012). "The hop phytoestrogen, 8-prenylnaringenin, reverses the ovariectomy-induced rise in skin temperature in an animal model of menopausal hot flushes.". Journal of Endocrinology. doi:10.1677/joe.1.06919. Retrieved 2013-04-07.
- ↑ 8.0 8.1 8.2 8.3 Overk, CR; Guo, J; Chadwick, LR; Lantvit, DD; Minassi, A; Appendino, G; Chen, SN; Lankin, DC; Farnsworth, NR; Pauli, GF; Van Breemen, RB; Bolton, JL (2008). "In vivo estrogenic comparisons of Trifolium pratense (red clover) Humulus lupulus (hops), and the pure compounds isoxanthohumol and 8-prenylnaringenin". Chemico-biological interactions 176 (1): 30–39. doi:10.1016/j.cbi.2008.06.005. PMC 2574795. PMID 18619951.
- ↑ Overk, C. R. et al. (August 2005). "Comparison of the In Vitro Estrogenic Activities of Compounds from Hops (Humulus lupulus) and Red Clover (Trifolium pratense)". J Agric Food Chem 53 (16): 6246–6253. doi:10.1021/jf050448p. PMC 1815392. PMID 16076101.
- ↑ Rad, Hümpel, Burggraaf; Hümpel; Schaefer; Schoemaker; Schleuning; Cohen; Burggraaf (September 1, 2006). "Pharmacokinetics and systemic endocrine effects of the phyto-oestrogen 8-prenylnaringenin after single oral doses to postmenopausal women". British Journal of Clinical Pharmacology 62 (3): 288–296. doi:10.1111/j.1365-2125.2006.02656.x. PMC 1885137. PMID 16934044.
- ↑ Bowe (November 2006). "The hop phytoestrogen, 8-prenylnaringenin, reverses the ovariectomy-induced rise in skin temperature in an animal model of menopausal hot flushes.". Journal of Endocrinology. doi:10.1677/joe.1.06919. Retrieved 2013-04-08.
- ↑ Bolca, Selin; Li, Jinghu; Nikolic, Dejan; Roche, Nathalie; Blondeel, Phillip; Possemiers, Sam; De Keukeleire, Denis; Bracke, Marc; Heyerick, Arne; Van Breemen, Richard B.; Depypere, Herman (2010). "Disposition of hop prenylflavonoids in human breast tissue". Molecular Nutrition & Food Research 54: S284–94. doi:10.1002/mnfr.200900519. PMC 3856213. PMID 20486208.
- ↑ S. R. Milligan, J. C. Kalita, V. Pocock, V. Van De Kauter, J. F. Stevens, M. L. Deinzer, H. Rong and D. De Keukeleire (December 2000). "The Endocrine Activities of 8-Prenylnaringenin and Related Hop (Humulus lupulus L.) Flavonoids" (PDF). Journal of Clinical Endocrinology & Metabolism 85 (12): 4912–4915. doi:10.1210/jc.85.12.4912. PMID 11134162.
- ↑ "Environmental 'hormones' wreck sperm". BBC News. July 2, 2002. Retrieved 2013-06-26.
- ↑ Breen, L. et al. (2009). "The effect of synthetic analogues of the phyto-oestrogen 8-prenylnaringenin on tail skin temperature in a rat hot flush model". The Physiological Society.
- ↑ Chadwick; Pauli; Farnsworth (July 1, 2005). "The pharmacognosy of Humulus lupulus L. (hops) with an emphasis on estrogenic properties". Phytomedicine. doi:10.1016/j.phymed.2004.07.006. Retrieved 2013-04-12.
| ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||