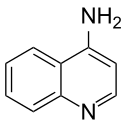
4-Aminoquinoline
 | |
 | |
| Names | |
|---|---|
| IUPAC name
quinolin-4-amine | |
| Identifiers | |
| 578-68-7 | |
| ChEMBL | ChEMBL58146 |
| ChemSpider | 61751 |
| |
| Jmol-3D images | Image |
| PubChem | 68476 |
| |
| UNII | GTE5P5L97N |
| Properties | |
| Molecular formula |
C9H8N2 |
| Molar mass | 144.17 g·mol−1 |
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| | |
| Infobox references | |
4-Aminoquinoline is a form of aminoquinoline with the amino group at the 4-position of the quinoline.
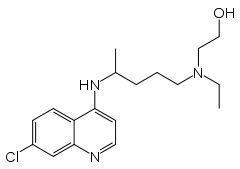
A variety of derivatives of 4-aminoquinoline are antimalarial agents useful in treating erythrocytic plasmodial infections. Examples include amodiaquine, chloroquine, and hydroxychloroquine.[1]
References
- ↑ Bray PG, Hawley SR, Ward SA (1996). "4-Aminoquinoline resistance of Plasmodium falciparum: insights from the study of amodiaquine uptake". Mol. Pharmacol. 50 (6): 1551–8. PMID 8967977.
External links
- Bourne SA, De Villiers K, Egan TJ (2006). "Three 4-aminoquinolines of antimalarial interest". Acta Crystallogr C 62 (Pt 2): o53–7. doi:10.1107/S0108270105041235. PMID 16456284.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||