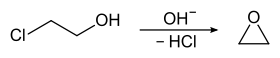2-Chloroethanol
| | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-Chloroethanol | |||
| Other names | |||
| Identifiers | |||
| 3DMet | B01042 | ||
| 878139 | |||
| 107-07-3 | |||
| ChEBI | CHEBI:28200 | ||
| ChEMBL | ChEMBL191244 | ||
| ChemSpider | 21106015 | ||
| EC number | 203-459-7 | ||
| 25389 | |||
| |||
| Jmol-3D images | Image | ||
| KEGG | C06753 | ||
| MeSH | Ethylene+Chlorohydrin | ||
| PubChem | 34 | ||
| RTECS number | KK0875000 | ||
| |||
| UNII | 753N66IHAN | ||
| UN number | 1135 | ||
| Properties | |||
| Molecular formula |
C2H5ClO | ||
| Molar mass | 80.51 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | faint, ether-like | ||
| Density | 1.201 g mL−1 | ||
| Melting point | −62.60 °C; −80.68 °F; 210.55 K | ||
| Boiling point | 127 °C; 260 °F; 400 K | ||
| miscible[2] | |||
| log P | −0.107 | ||
| Vapor pressure | 700 Pa (at 20 °C) | ||
| Refractive index (nD) |
1.441 | ||
| Thermochemistry | |||
| Std enthalpy of combustion (ΔcH |
−1.1914 MJ mol−1 | ||
| Hazards | |||
| GHS pictograms |   | ||
| GHS signal word | DANGER | ||
| H226, H300, H310, H330 | |||
| P260, P280, P284, P301+310, P302+350 | |||
| EU Index | 603-028-00-7 | ||
| EU classification | | ||
| R-phrases | R26/27/28 | ||
| S-phrases | (S1/2), S45 | ||
| NFPA 704 | |||
| Flash point | 55 °C (131 °F; 328 K) | ||
| 425 °C (797 °F; 698 K) | |||
| Explosive limits | 5–16% | ||
| LD50 (Median lethal dose) |
67 mg kg−1 (dermal, rabbit) | ||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
TWA 5 ppm (16 mg/m3) [skin][2] | ||
| REL (Recommended) |
C 1 ppm (3 mg/m3) [skin][2] | ||
| IDLH (Immediate danger) |
7 ppm[2] | ||
| Related compounds | |||
| Related compounds |
|||
| Except where noted otherwise, data is given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |||
| | |||
| Infobox references | |||
2-Chloroethanol is an organochlorine compound with the formula HOCH2CH2Cl. This colorless liquid has a pleasant ether-like odor. It is miscible with water. The molecule is bifunctional, consisting of both an alkyl chloride and an alcohol functional groups.
Synthesis and applications
2-Chloroethanol is produced by treating ethylene with hypochlorous acid:
2-Chloroethanol was once produced on a large scale as a precursor to ethylene oxide:
This application has been supplanted by the greener direct oxidation of ethylene. Otherwise chloroethanol is used in a number of specialized applications.[3] Several dyes are prepared by the alkylation of aniline derivatives with chloroethanol.[4] It is a building block in the production of pharmaceuticals, biocides and plasticizers. It is also used for manufacture of thiodiglycol. It is a solvent for cellulose acetate and ethyl cellulose, textile printing dyes, in dewaxing, refining of rosin, extraction of pine lignin, and the cleaning of machines.
Environmental aspects
Chloroethanol is a metabolite in the degradation of 1,2-dichloroethane. The alcohol is then further oxidized via chloroacetaldehyde to chloroacetate. This metabolic pathway is topical since billions of kilograms of 1,2-dichloroethane are processed annually as a precursor to vinyl chloride.[5]
Safety
2-Chloroethanol is toxic with an LD50 of 89 mg/kg in rats. Like most organochlorine compounds, chloroethanol combusts to yield hydrogen chloride and phosgene.
In regards to dermal exposure to 2-chloroethanol, the Occupational Safety and Health Administration has set a permissible exposure limit of 5 ppm (16 mg/m3) over an eight-hour time-weighted average, while the National Institute for Occupational Safety and Health has a more protective recommended exposure limit of a 1 ppm (3 mg/m3) exposure ceiling.[6]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 1.10 1.11 Depositor-supplied synonyms for CID 34
- ↑ 2.0 2.1 2.2 2.3 "NIOSH Pocket Guide to Chemical Hazards #0268". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Gordon Y. T. Liu, W. Frank Richey, Joanne E. Betso "Chlorohydrins" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a06_565
- ↑ Roderich Raue and John F. Corbett "Nitro and Nitroso Dyes" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a17_383
- ↑ 1. Janssen, D. B.; van der Ploeg, J. R. and Pries, F., "Genetics and Biochemistry of 1,2-Dichloroethane Degradation", Biodegradation, 1994, volume 5, pp. 249-57.doi:10.1007/BF00696463
- ↑ CDC - NIOSH Pocket Guide to Chemical Hazards