-yne
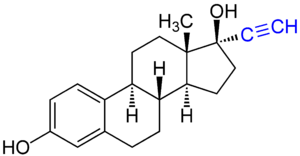
Ethinyl group (highlighted
blue) as part of a large molecule (ethinylestradiol).
[1]The suffix -yne is used in IUPAC chemical nomenclature to convey specific information regarding the presence of a triple bond within the parent compound. -yne is mainly used within organic nomenclature, however inorganic compounds containing unsaturation in the form of triple bond(s), are accommodated in substitutive nomenclature by the same methods used with alkynes i.e. the name of the
corresponding saturated hydride is modified by replacing the '-ane’ ending with ‘-yne’. ‘-diyne’ is used when there are two triple bonds, and so on. The position of unsaturation is indicated by a numerical locant immediately preceding the '-yne' suffix, or 'locants' in the case of multiple triple bonds. Locants are chosen to be as low as possible. '-yne' is also used as an infix to name substituent groups that are triply bound to the parent compound.
Sometimes a number between hyphens is inserted before it to state which atoms the triple bond is between. This suffix arose as a collapsed form of the end of the word "acetylene". The final "-e" disappears if it is followed by another suffix that starts with a vowel.[2]
References
- ↑ Europäisches Arzneibuch, 6. Ausgabe, Deutscher Apotheker Verlag Stuttgart 2008, ISBN 978-3-7692-3962-1, pp. 2503–2504.
- ↑ The Commission on the Nomenclature of Organic Chemistry (1971) [1958 (A: Hydrocarbons, and B: Fundamental Heterocyclic Systems), 1965 (C: Characteristic Groups)]. Nomenclature of Organic Chemistry (3rd ed.). London: Butterworths. ISBN 0-408-70144-7.
See also
 |
Look up -yne in Wiktionary, the free dictionary. |
