Xenon tetrafluoride
| Xenon tetrafluoride | |
|---|---|
 | |
 |
 |
| IUPAC name Xenon tetrafluoride | |
| Identifiers | |
| CAS number | 13709-61-0 |
| PubChem | 123324 |
| ChemSpider | 109927 |
| Jmol-3D images | {{#if:F[Xe](F)(F)F|Image 1 |
| |
| |
| Properties | |
| Molecular formula | XeF 4 |
| Molar mass | 207.2836 g mol−1 |
| Appearance | White solid |
| Density | 4.040 g cm−3, solid |
| Melting point | 117 °C sublimes (390 K)[1] |
| Solubility in water | Reacts |
| Structure | |
| Coordination geometry |
D4h |
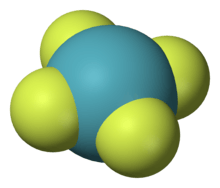
| Molecular shape | square planar |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std enthalpy of formation ΔfH |
−251 kJ·mol−1[2] |
| Standard molar entropy S |
146 J·mol−1·K−1[2] |
| Hazards | |
| Flash point | ? °C |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Xenon tetrafluoride is a chemical compound with chemical formula XeF
4. It was the first discovered binary compound of a noble gas.[3] It is produced by the chemical reaction of xenon with fluorine, F
2, according to the chemical equation:[4][5]
- Xe + 2 F
2 → XeF
4
This reaction is exothermic, releasing an energy of 251 kJ/mol of xenon.[3]
Xenon tetrafluoride is a colorless crystalline substance under ordinary conditions. Its crystalline structure was determined by both NMR spectroscopy and X-ray crystallography in 1963.[6][7] The structure is square planar, as has been confirmed by neutron diffraction studies,[8] and is justified by VSEPR theory because xenon has two lone pairs of electrons above and below the plane of the molecule.
Xenon tetrafluoride sublimes at a temperature of 115.7 °C (240.26 °F).
The formation of xenon tetrafluoride, like the other xenon fluorides, is exergonic. They are stable at normal temperatures and pressures. All of them readily react with water, releasing pure xenon gas, hydrogen fluoride, and molecular oxygen. This reaction occurs in slightly moist air; hence, all xenon fluorides must be kept in anhydrous atmospheres.
Synthesis
Xenon tetrafluoride is produced by heating a mixture of xenon and fluorine in a 1:3 ratio in a nickel container to 400 °C. Some xenon hexafluoride, XeF
6, is also produced, and this production is increased with an increased fluorine concentration in the input mixture.[9] The nickel is not a catalyst for this reaction; nickel containers are used because they react with fluorine to form a protective, non-peeling layer of nickel fluoride NiF
2 on their interior surfaces.
Chemistry
Xenon tetrafluoride is hydrolyzed by water at low temperatures to form elemental xenon, oxygen, hydrofluoric acid, and aqueous xenon trioxide.[10]
Reaction with tetramethylammonium fluoride forms tetramethylammonium pentafluoroxenate, which contains the pentagonal XeF−
5 anion. The XeF−
5 anion is also formed by reaction with caesium fluoride:[11]
- CsF + XeF
4 → CsXeF
5
Reaction with bismuth pentafluoride (BiF
5) forms the XeF+
3 cation:[12]
- BiF
5 + XeF
4 → XeF3BiF6
The XeF+
3 cation has also been identified in the salt XeF3Sb2F11 by NMR spectroscopy.[13]
At 400 °C, XeF
4 reacts with xenon gas to form XeF
2:[9]
- XeF4 + Xe → 2 XeF2
The reaction of xenon tetrafluoride with platinum yields platinum tetrafluoride (PtF
4) and xenon gas:[9]
- XeF4 + Pt → PtF4 + Xe
Applications
Xenon tetrafluoride is used as a decomposition agent of silicone rubber for analysing trace metal impurities in the rubber. XeF
4 reacts with the silicone structure that makes up the backbone of silicone rubber to form simple gaseous products, leaving behind any content of metal impurities.[14]
References
- ↑ Arnold F. Holleman; Egon Wiberg (2001). Nils Wiberg, ed. Inorganic chemistry. translated by Mary Eagleson, William Brewer. Academic Press. p. 394. ISBN 0-12-352651-5.
- ↑ 2.0 2.1 Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A23. ISBN 0-618-94690-X.
- ↑ 3.0 3.1 Zumdahl (2007). Chemistry. Boston: Houghton Mifflin. p. 243. ISBN 0-618-52844-X.
- ↑ Claassen, H. H.; Selig, H.; Malm, J. G. (1962). "Xenon Tetrafluoride". J. Am. Chem. Soc. 84 (18): 3593. doi:10.1021/ja00877a042.
- ↑ C. L. Chernick, H. H. Claassen, P. R. Fields 1, H. H. Hyman, J. G. Malm, W. M. Manning, M. S. Matheson, L. A. Quarterman, F. Schreiner, H. H. Selig, I. Sheft, S. Siegel, E. N. Sloth, L. Stein, M. H. Studier, J. L. Weeks, and M. H. Zirin (1962). "Fluorine Compounds of Xenon and Radon". Science 138 (3537): 136–138. Bibcode:1962Sci...138..136C. doi:10.1126/science.138.3537.136. PMID 17818399.
- ↑ Thomas H. Brown, E. B. Whipple, and Peter H. Verdier (1963). "Xenon Tetrafluoride: Fluorine-19 High-Resolution Magnetic Resonance Spectrum". Science 140 (3563): 178. Bibcode:1963Sci...140..178B. doi:10.1126/science.140.3563.178. PMID 17819836.
- ↑ James A. Ibers and Walter C. Hamilton (1963). "Xenon Tetrafluoride: Crystal Structure". Science 139 (3550): 106–107. Bibcode:1963Sci...139..106I. doi:10.1126/science.139.3550.106. PMID 17798707.
- ↑ Burns, John H.; Agron, P. A.; Levy, Henri A (1963). "Xenon Tetrafluoride Molecule and Its Thermal Motion: A Neutron Diffraction Study". Science 139 (3560): 1208–1209. Bibcode:1963Sci...139.1208B. doi:10.1126/science.139.3560.1208. PMID 17757912.
- ↑ 9.0 9.1 9.2 Allen J. Bard; Roger Parsons; Joseph Jordan; International Union of Pure and Applied Chemistry (1985). Standard Potentials in Aqueous Solution. CRC Press. pp. 767–768. ISBN 0-8247-7291-1.
- ↑ Williamson; Koch, C. W. (Mar 1963). "Xenon Tetrafluoride: Reaction with Aqueous Solutions". Science 139 (3559): 1046–1047. Bibcode:1963Sci...139.1046W. doi:10.1126/science.139.3559.1046. ISSN 0036-8075. PMID 17812981.
- ↑ Charlie Harding; David Arthur Johnson; Rob Janes (2002). Elements of the p block (Volume 9 of Molecular world). Royal Society of Chemistry. p. 93. ISBN 0-85404-690-9.
- ↑ Hitomi Suzuki; Yoshihiro Matano (2001). Organobismuth chemistry. Elsevier. p. 8. ISBN 0-444-20528-4.
- ↑ Gillespie, R. J.; B. Landa; G. J. Schrobilgen (1971). "Trifluoroxenon(IV)µ-fluoro-bispentafluoroantimonate(V): the XeF+
3 cation". Journal of the Chemical Society D: Chemical Communications (23): 1543–1544. doi:10.1039/C29710001543. - ↑ Rigin, V.; Skvortsov, N. K.; Rigin, V. V. (March 1997). "Xenon tetrafluoride as a decomposition agent for silicone rubber for isolation and atomic emission spectrometric determination of trace metals". Analytica Chimica Acta 340 (1–3): 1–3. doi:10.1016/S0003-2670(96)00563-6.
External links
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||