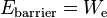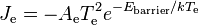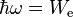Work function
In solid-state physics, the work function (sometimes spelled workfunction) is the minimum thermodynamic work (i.e. energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" means that the final electron position is far from the surface on the atomic scale, but still too close to the solid to be influenced by ambient electric fields in the vacuum. The work function is not a characteristic of a bulk material, but rather a property of the surface of the material (depending on crystal face and contamination).
Definition
The work function W for a given surface is defined by the difference[1]
where −e is the charge of an electron, ϕ is the electrostatic potential in the vacuum nearby the surface, and EF is the Fermi level (electrochemical potential of electrons) inside the material. The term −eϕ is the energy of an electron at rest in the vacuum nearby the surface, and the meaning of the term −EF is the thermodynamic work required to remove an electron from the material to a state of zero total energy. In words, the work function is thus defined as the thermodynamic work required to remove an electron from the material to a state at rest in the vacuum nearby the surface.

In practice, one directly controls EF by the voltage applied to the material through electrodes, and the work function is generally a fixed characteristic of the surface material. Consequently this means that when a voltage is applied to a material, the electrostatic potential ϕ produced in the vacuum will be somewhat lower than the applied voltage, the difference depending on the work function of the material surface. Rearranging the above equation, one has
where V = −EF/e is the voltage of the material (as measured by a voltmeter, through an attached electrode), relative to an electrical ground that is defined as having zero Fermi level. The fact that ϕ depends on material surface means that the space between two dissimilar conductors will have a built-in electric field, even when those conductors are in total equilibrium with each other (electrically shorted to each other, and with equal temperatures). An example of this situation is depicted in the adjacent figure. As described in the next section, these built-in vacuum electric fields can have important consequences in some cases.
Applications
- Non-uniform vacuum in vacuum chambers: An important implication of the work function is that there will be spatial variations in the vacuum electrostatic potential inside a vacuum chamber, if it is not lined with a material of uniform work function. These equilibrium variations in ϕ (known as Volta potentials, contact potential differences, patch fields, or patch potentials) can disrupt sensitive apparatus that rely on a perfectly uniform vacuum. For example, the Gravity Probe B experiment was significantly impacted by variations in W (and thus ϕ) over the surface of a free spinning gyroscope, as the resulting electric dipole torques resulted in precession and slowing due to dissipation by the induced currents. Critical apparatus may have surfaces covered with molybdenum, which shows low variations in work function between different crystal faces.[2]
- Thermionic emission: In thermionic electron guns, the work function and temperature of the hot cathode are critical parameters in determining the amount of current that can be emitted. Tungsten, the common choice for vacuum tube filaments, can survive to high temperatures but its emission is somewhat limited due to its relatively high work function (approximately 4.5 eV). By coating the tungsten with a substance of lower work function (e.g., thorium or barium oxide), the emission can be greatly increased. This prolongs the lifetime of the filament by allowing operation at lower temperatures (for more information, see hot cathode).
- Conductor-insulator work functions: In many ways an insulator behaves analogously to the vacuum, with the bottom of the conduction band in the insulator playing the role of the vacuum level −eϕ. The "metal–insulator work function" of a given metal–insulator interface differs greatly from the vacuum work function of the metal, however the difference of this "work function" between two materials separated by an insulator may be equal to the vacuum work function difference of those materials.[citation needed] In insulated gate electronic devices (such as MOSFETs) the built-in electric field in the insulator influences the threshold voltage required to form an inversion layer, and is strongly influenced by the work function of the gate material.[3]
- Band bending in semiconductors: In conductor-conductor junctions where at least one conductor is a semiconductor, there are heuristic rules that predict the degree of band bending based on the thought experiment of two materials coming together in vacuum, such that the materials must equalize their work function upon contact. For a semiconductor-semiconductor junction the resulting heuristic rule is known as Anderson's rule and is reasonably accurate.[4] For a metal-semiconductor junction the resulting heuristic rule is known as the Schottky-Mott rule and gives poor accuracy, due to a phenomenon known as Fermi level pinning.[5]
- Contact electrification: If two conducting surfaces are moved relative to each other, and there is potential difference in the space between them, then an electrical current will be driven. This is because the surface charge on a conductor depends on the magnitude of the electric field, which in turn depends on the distance between the surfaces. The externally observed electrical effects are largest when the conductors are separated by the smallest distance without touching (once brought into contact, the charge will instead flow internally through the junction between the conductors). Since two conductors in equilibrium can have a built-in potential difference due to work function differences, this means that bringing dissimilar conductors into contact, or pulling them apart, will drive electrical currents. These contact currents can damage sensitive microelectronic circuitry and occur even when the conductors would be grounded in the absence of motion.[6]
Measurement
Certain physical phenomena are highly sensitive to the value of the work function. The observed data from these effects can be fitted to simplified theoretical models, allowing one to extract a value of the work function. These phenomenologically extracted work functions may be slightly different from the thermodynamic definition given above. For inhomogeneous surfaces, the work function varies from place to place, and different methods will yield different values of the typical "work function" as they average or select differently among the microscopic work functions.[7]
Many techniques have been developed based on different physical effects to measure the electronic work function of a sample. One may distinguish between two groups of experimental methods for work function measurements: absolute and relative.
- Absolute methods employ electron emission from the sample induced by photon absorption (photoemission), by high temperature (thermionic emission), due to an electric field (field electron emission), or using electron tunnelling.
- Relative methods make use of the contact potential difference between the sample and a reference electrode. Experimentally, either an anode current of a diode is used or the displacement current between the sample and reference, created by an artificial change in the capacitance between the two, is measured (the Kelvin Probe method, Kelvin probe force microscope).
Methods based on thermionic emission
The work function is important in the theory of thermionic emission, where thermal fluctuations provide enough energy to "evaporate" electrons out of a hot material (called the 'emitter') into the vacuum. If these electrons are absorbed by another, cooler material (called the collector) then a measurable electric current will be observed. Thermionic emission can be used to measure the work function of both the hot emitter and cold collector.
Work function of hot electron emitter

In order to move from the hot emitter to the vacuum, the electrons must overcome an energy barrier
determined simply by the thermionic work function of the emitter. If an electric field is applied into the surface of the emitter, then the escaping electrons will all be accelerated away from the emitter and absorbed into whichever material is applying the electric field. According to Richardson's law the emitted current density (per unit area of emitter), Je (A/m2), is related to the absolute temperature Te of the emitter by the equation:
where k is the Boltzmann constant and the proportionality constant Ae is the Richardson's constant of the emitter. In this case, the dependence of Je on Te can be fitted to yield We.
Work function of cold electron collector

The same construction can be used to instead measure the work function in the collector. If an electric field is applied out of the emitter instead, the electrons must overcome an additional barrier before reaching their destination (the collector). This collector barrier depends on the work function of the collector, as well as any additional applied voltages:[8]
where Wc is the collector's thermionic work function, ΔVce is the applied collector–emitter voltage, and ΔVS is the Seebeck voltage in the hot emitter (the influence of ΔVS is often omitted). The resulting current density Jc through the collector (per unit of collector area) is again given by Richardson's Law, except now
where Ac is the Richardson constant of the collector. In this case, the dependence of Jc on Te can be fitted to yield Wc.
This retarding potential (or retarding diode) method is one of the simplest and oldest methods of measuring work functions, and is advantageous since the collector need not be heated.
Methods based on photoemission

The photoelectric work function is the minimum photon energy required to liberate an electron from a substance, in the photoelectric effect.
If the photon's energy is greater than the substance's work function, photoelectric emission occurs and the electron is liberated from the surface.
Similar to the thermionic case described above, the liberated electrons can be extracted into a collector and produce a detectable current, if an electric field is applied into the surface of the emitter.
Excess photon energy results in a liberated electron with non-zero kinetic energy.
It is expected that the minimum photon energy  required to liberate an electron (and generate a current) is
required to liberate an electron (and generate a current) is
where We is the work function of the emitter.
Photoelectric measurements require a great deal of care, as an incorrectly designed experimental geometry can result in an erroneous measurement of work function.[7] This may be responsible for the large variation in work function values in scientific literature. Moreover, the minimum energy can be misleading in materials where there are no actual electron states at the Fermi level that are available for excitation. For example, in a semiconductor the minimum photon energy would actually correspond to the valence band edge rather than work function.[9]
Of course, the photoelectric effect may be used in the retarding mode, as with the thermionic apparatus described above. In the retarding case, the dark collector's work function is measured instead.
Kelvin probe method

The Kelvin probe technique relies on the detection of an electric field (gradient in ϕ) between a sample material and probe material. The electric field can be varied by the voltage ΔVsp that is applied to the sample relative to the probe. If the voltage is chosen such that the electric field is eliminated (the flat vacuum condition), then
Since the experimenter controls and knows ΔVsp, then finding the flat vacuum condition gives directly the work function difference between the two materials. The only question is, how to detect the flat vacuum condition? Typically, the electric field is detected by varying the distance between the sample and probe. When the distance is changed but ΔVsp is held constant, a current will flow due to the change in capacitance. This current is proportional to the vacuum electric field, and so when the electric field is neutralized no current will flow.
Although the Kelvin probe technique only measures a work function difference, it is possible to obtain an absolute work function by first calibrating the probe against a reference material (with known work function) and then using the same probe to measure a desired sample. The Kelvin probe technique can be used to obtain work function maps of a surface with extremely high spatial resolution, by using a sharp tip for the probe (see Kelvin probe force microscope).
Work functions of elements[10]
Below is a table of work function values for various elements. Note that the work function depends on the configurations of atoms at the surface of the material. For example, on polycrystalline silver the work function is 4.26 eV, but on silver crystals it varies for different crystal faces as (100) face: 4.64 eV, (110) face: 4.52 eV, (111) face: 4.74 eV.[11] Ranges for typical surfaces are shown in the table below.
| Ag | 4.26 – 4.74 | Al | 4.06 – 4.26 | As | 3.75 |
| Au | 5.1 – 5.47 | B | ~4.45 | Ba | 2.52 – 2.7 |
| Be | 4.98 | Bi | 4.31 | C | ~5 |
| Ca | 2.87 | Cd | 4.08 | Ce | 2.9 |
| Co | 5 | Cr | 4.5 | Cs | 2.14 |
| Cu | 4.53 – 5.10 | Eu | 2.5 | Fe: | 4.67 – 4.81 |
| Ga | 4.32 | Gd | 2.90 | Hf | 3.9 |
| Hg | 4.475 | In | 4.09 | Ir | 5.00 – 5.67 |
| K | 2.29 | La | 3.5 | Li | 2.9 |
| Lu | ~3.3 | Mg | 3.66 | Mn | 4.1 |
| Mo | 4.36 – 4.95 | Na | 2.36 | Nb | 3.95 – 4.87 |
| Nd | 3.2 | Ni | 5.04 – 5.35 | Os | 5.93 |
| Pb | 4.25 | Pd | 5.22 – 5.6 | Pt | 5.12 – 5.93 |
| Rb | 2.261 | Re | 4.72 | Rh | 4.98 |
| Ru | 4.71 | Sb | 4.55 – 4.7 | Sc | 3.5 |
| Se | 5.9 | Si | 4.60 – 4.85 | Sm | 2.7 |
| Sn | 4.42 | Sr | ~2.59 | Ta | 4.00 – 4.80 |
| Tb | 3.00 | Te | 4.95 | Th | 3.4 |
| Ti | 4.33 | Tl | ~3.84 | U | 3.63 – 3.90 |
| V | 4.3 | W | 4.32 – 5.22 | Y | 3.1 |
| Yb | 2.60 [12] | Zn | 3.63 – 4.9 | Zr | 4.05 |
Physical factors that determine the work function
Due to the complications described in the modelling section below, it is difficult theoretically predict the work function with accuracy. Various trends have however been identified. The work function tends to be smaller for metals with an open lattice, and larger for metals in which the atoms are closely packed. It is somewhat higher on dense crystal faces than open crystal faces, also depending on surface reconstructions for the given crystal face.
Surface dipole
The work function is not simply dependent on the "internal vacuum level" inside the material (i.e., its average electrostatic potential), because of the formation of an atomic-scale electric double layer at the surface.[2] This surface electric dipole gives a jump in the electrostatic potential between the material and the vacuum.
A variety of factors are responsible for the surface electric dipole. Even with a completely clean surface, the electrons can spread slightly into the vacuum, leaving behind a slightly positively charged layer of material. This primarily occurs in metals, where the bound electrons do not encounter a hard wall potential at the surface but rather a gradual ramping potential due to image charge attraction. The amount of surface dipole depends on the detailed layout of the atoms at the surface of the material, leading to the variation in work function for different crystal faces.
Doping and electric field effect (semiconductors)
In a semiconductor, the work function is sensitive to the doping level at the surface of the semiconductor. Since the doping near the surface can be also be controlled by electric fields, the work function of a semiconductor is also sensitive to the electric field in the vacuum.
The reason for the dependence is that, typically, the vacuum level and the conduction band edge retain a fixed spacing independent of doping. This spacing is called the electron affinity (note that this has a different meaning than the electron affinity of chemistry); in silicon for example the electron affinity is 4.05 eV.[13] If the electron affinity EEA and the surface's band-referenced Fermi level EF-EC are known, then the work function is given by
where EC is taken at the surface.
From this one might expect that by doping the bulk of the semiconductor, the work function can be tuned. In reality, however, the energies of the bands near the surface are often pinned to the Fermi level, due to the influence of surface states.[14] If there is a large density of surface states, then the work function of the semiconductor will show a very weak dependence on doping or electric field.[15]
Theoretical models of metal work functions
Theoretical modelling of the work function is difficult, as an accurate model requires a careful treatment of both electronic many body effects and surface chemistry; both of these topics are already complex in their own right.
One of the earliest successful models for metal work function trends was the jellium model,[16] which allowed for oscillations in electronic density nearby the abrupt surface (these are similar to Friedel oscillations) as well as the tail of electron density extending outside the surface. This model showed why the density of conduction electrons (as represented by the Wigner-Seitz radius rs) is an important parameter in determining work function.
The jellium model is only a partial explanation, as its predictions still show significant deviation from real work functions. More recent models have focussed on including more accurate forms of electron exchange and correlation effects, as well as including the crystal face dependence (this requires the inclusion of the actual atomic lattice, something that is neglected in the jellium model).[2][17]
References
- ↑ Kittel, Charles. Introduction to Solid State Physics, 7th Edition. Wiley.
- ↑ 2.0 2.1 2.2 http://venables.asu.edu/qmms/PROJ/metal1a.html
- ↑ Lin, R.; Qiang Lu; Ranade, P.; Tsu-Jae King; Chenming Hu (2002). "An adjustable work function technology using Mo gate for CMOS devices". IEEE Electron Device Letters 23: 49. doi:10.1109/55.974809.
- ↑ Debbar, N.; Biswas, Dipankar; Bhattacharya, Pallab (1989). "Conduction-band offsets in pseudomorphic InxGa1-xAs/Al0.2Ga0.8As quantum wells (0.07≤x≤0.18) measured by deep-level transient spectroscopy". Physical Review B 40 (2): 1058. doi:10.1103/PhysRevB.40.1058.
- ↑ http://academic.brooklyn.cuny.edu/physics/tung/Schottky/systematics.htm
- ↑ Thomas Iii, S. W.; Vella, S. J.; Dickey, M. D.; Kaufman, G. K.; Whitesides, G. M. (2009). "Controlling the Kinetics of Contact Electrification with Patterned Surfaces". Journal of the American Chemical Society 131 (25): 8746–8747. doi:10.1021/ja902862b. PMID 19499916.
- ↑ 7.0 7.1 Helander, M. G.; Greiner, M. T.; Wang, Z. B.; Lu, Z. H. (2010). "Pitfalls in measuring work function using photoelectron spectroscopy". Applied Surface Science 256 (8): 2602. doi:10.1016/j.apsusc.2009.11.002.
- ↑ "Thermionic Energy Conversion"
- ↑ http://www.virginia.edu/ep/SurfaceScience/PEE.html
- ↑ CRC Handbook of Chemistry and Physics version 2008, p. 12–114.
- ↑ Dweydari, A. W.; Mee, C. H. B. (1975). "Work function measurements on (100) and (110) surfaces of silver". Physica Status Solidi (a) 27: 223. doi:10.1002/pssa.2210270126.
- ↑ Nikolic, M. V.; Radic, S. M.; Minic, V.; Ristic, M. M. (February 1996). "The dependence of the work function of rare earth metals on their electron structure". Microelectronics Journal 27 (1): 93–96. doi:10.1016/0026-2692(95)00097-6. ISSN 0026-2692. Retrieved 2009-09-22.
- ↑ http://www.virginiasemi.com/pdf/generalpropertiessi62002.pdf
- ↑ http://academic.brooklyn.cuny.edu/physics/tung/Schottky/surface.htm
- ↑ Bardeen, J. (1947). "Surface States and Rectification at a Metal Semi-Conductor Contact". Physical Review 71 (10): 717. Bibcode:1947PhRv...71..717B. doi:10.1103/PhysRev.71.717.
- ↑ Lang, N.; Kohn, W. (1971). "Theory of Metal Surfaces: Work Function". Physical Review B 3 (4): 1215. Bibcode:1971PhRvB...3.1215L. doi:10.1103/PhysRevB.3.1215.
- ↑ Kiejna, A.; Wojciechowski, K.F. (1996). Metal Surface Electron Physics. Elsevier. ISBN 9780080536347.
Further reading
- Ashcroft; Mermin (1976). Solid State Physics. Thomson Learning, Inc.
- Goldstein, Newbury; et al. (2003). Scanning Electron Microscopy and X-Ray Microanalysis. New York: Springer.
For a quick reference to values of work function of the elements:
- Michaelson, Herbert B. (1977). "The work function of the elements and its periodicity". J. Appl. Phys. 48: 4729. Bibcode:1977JAP....48.4729M. doi:10.1063/1.323539.
External links
- Work function of polymeric insulators (Table 2.1)
- Work function of diamond and doped carbon
- Work functions of common metals
- Work functions of various metals for the photoelectric effect
- Physics of free surfaces of semiconductors
*Some of the work functions listed on these sites do not agree!*
| ||||||||||||||