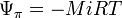Water potential
Water potential is the potential energy of water per unit volume relative to pure water in reference conditions. Water potential quantifies the tendency of water to move from one area to another due to osmosis, gravity, mechanical pressure, or matrix effects such as capillary action (which is caused by surface tension). Water potential has proved especially useful in understanding water movement within plants, animals, and soil. Water potential is typically expressed in potential energy per unit volume and very often is represented by the Greek letter 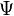 .
.
Water potential integrates a variety of different potential drivers of water movement, which may operate in the same or different directions. Within complex biological systems, it is common for many potential factors to be important. For example, the addition of solutes to water lowers the water's potential (makes it more negative), just as the increase in pressure increases its potential (makes it more positive). If there is no restriction on flow, water will move from an area of higher water potential to an area that has a lower water potential. One very common example is water that contains a dissolved salt, like sea water or the solution within living cells. These solutions typically have negative water potentials, relative to the pure water reference. If there is no restriction on flow, water molecules will proceed from the locus of pure water to the more negative water potential of the solution; flow proceeds until the difference in solute potential is balanced by another force, for example, pressure potential.
Components of water potential
Many different factors may affect the total water potential, and the sum of these potentials determines the overall water potential and the direction of water flow:
where:
 is the reference correction,
is the reference correction, is the solute potential,
is the solute potential,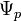 is the pressure component,
is the pressure component,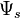 is the gravimetric component,
is the gravimetric component,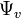 is the potential due to humidity, and
is the potential due to humidity, and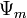 is the potential due to matrix effects (e.g., fluid cohesion and surface tension.)
is the potential due to matrix effects (e.g., fluid cohesion and surface tension.)
All of these factors are quantified as potential energies per unit volume, and different subsets of these terms may be used for particular applications (e.g., plants or soils). Different conditions are also defined as reference depending on the application: for example, in soils, the reference condition is typically defined as pure water at the soil surface.
Pressure Potential
Pressure potential is based on mechanical pressure, and is an important component of the total water potential within plant cells. Pressure potential increases as water enters a cell. As water passes through the cell wall and cell membrane, it increases the total amount of water present inside the cell, which exerts an outward pressure that is opposed by the structural rigidity of the cell wall. By creating this pressure, the plant can maintain turgor, which allows the plant to keep its rigidity. Without turgor, plants lose structure and wilt.
The pressure potential in a living plant cell is usually positive. In plasmolysed cells, pressure potential is almost zero. Negative pressure potentials occur when water is pulled through an open system such as a plant xylem vessel. Withstanding negative pressure potentials (frequently called tension) is an important adaptation of xylem. This tension can be measured empirically using the Pressure bomb.
Osmotic potential
Pure water is usually defined as having an osmotic potential (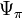 ) of zero, and in this case, solute potential can never be positive. The relationship of solute concentration (in molarity) to solute potential is given by the van 't Hoff equation:
) of zero, and in this case, solute potential can never be positive. The relationship of solute concentration (in molarity) to solute potential is given by the van 't Hoff equation:
where  is the concentration in molarity of the solute,
is the concentration in molarity of the solute,  is the van 't Hoff factor, the ratio of amount of particles in solution to amount of formula units dissolved,
is the van 't Hoff factor, the ratio of amount of particles in solution to amount of formula units dissolved,  is the ideal gas constant, and
is the ideal gas constant, and  is the absolute
temperature.
is the absolute
temperature.
For example, when a solute is dissolved in water, water molecules are less likely to diffuse away via osmosis than when there is no solute. A solution will have a lower and hence more negative water potential than that of pure water. Furthermore, the more solute molecules present, the more negative the solute potential is.
Osmotic potential has important implications for many living organisms. If a living cell is surrounded by a more concentrated solution, the cell will tend to lose water to the more negative water potential (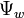 ) of the surrounding environment. This can be the case for marine organisms living in sea water and halophytic plants growing in saline environments. In the case of a plant cell, the flow of water out of the cell may eventually cause the plasma membrane to pull away from the cell wall, leading to plasmolysis. Most plants, however, have the ability to increase solute inside the cell to drive the flow of water into the cell and maintain turgor.
) of the surrounding environment. This can be the case for marine organisms living in sea water and halophytic plants growing in saline environments. In the case of a plant cell, the flow of water out of the cell may eventually cause the plasma membrane to pull away from the cell wall, leading to plasmolysis. Most plants, however, have the ability to increase solute inside the cell to drive the flow of water into the cell and maintain turgor.
This effect can be used to power an osmotic power plant.[2]
A soil solution also experiences osmotic potential. The osmotic potential is made possible due to the presence of both inorganic and organic solutes in the soil solution. As water molecules increasingly clump around solute ions or molecules, the freedom of movement, and thus the potential energy, of the water is lowered. As the concentration of solutes is increased, the osmotic potential of the soil solution is reduced. Since water has a tendency to move toward lower energy levels, water will want to travel toward the zone of higher solute concentrations. Although, liquid water will only move in response to such differences in osmotic potential if a semipermeable membrane exists between the zones of high and low osmotic potential. A semipermeable membrane is necessary because it allows water through its membrane while preventing solutes from moving through its membrane. If no membrane is present, movement of the solute, rather than of the water, largely equalizes concentrations.
Since regions of soil are usually not divided by a semipermeable membrane, the osmotic potential typically has a negligible influence on the mass movement of water in soils. On the other hand, osmotic potential has an extreme influence on the rate of water uptake by plants. If soils are high in soluble salts, the osmotic potential is likely to be lower in the soil solution than in the plant root cells. In such cases, the soil solution would severely restrict the rate of water uptake by plants. In salty soils, the osmotic potential of soil water may be so low that the cells in young seedlings start to collapse (plasmolyze).
Matrix potential (Matric potential)
When water is in contact with solid particles (e.g., clay or sand particles within soil), adhesive intermolecular forces between the water and the solid can be large and important. The forces between the water molecules and the solid particles in combination with attraction among water molecules promote surface tension and the formation of menisci within the solid matrix. Force is then required to break these menisci. The magnitude of matrix potential depends on the distances between solid particles—the width of the menisci (see also capillary action)--and the chemical composition of the solid matrix.
In many cases, matrix potential can be relatively large in comparison to the other components of water potential discussed above. Matrix potential markedly reduces the energy state of water near particle surfaces. Although water movement due to matrix potential may be slow, it is still extremely important in supplying water to plant roots and in engineering applications. The matrix potential is always negative because the water attracted by the soil matrix has an energy state lower than that of pure water. Matrix potential only occurs in unsaturated soil above the water table. If the matrix potential approaches a value of zero, nearly all soil pores are completely filled with water, i.e. fully saturated and at maximum retentive capacity. The matrix potential can vary considerably among soils. In the case that water drains into less-moist soil zones of similar porosity, the matrix potential is generally in the range of -10 to -30 kPa.
It is worth noting that matrix potentials are very important for plant water relations. Strong (very negative) matrix potentials bind water to soil particles within very dry soils. Plants then create even more negative matrix potentials within tiny pores in the cell walls of their leaves to extract water from the soil and allow physiological activity to continue through dry periods. Germinating seeds have a very negative matrix potentials, creating water uptake in even somewhat dry soils and hydrates the dry seed.
Soil Water Potential
At a potential of 0 kPa, soil is in a state of saturation. At saturation, all soil pores are filled with water, and water typically drains from large pores by gravity. At a potential of -33 kPa (-10 kPa for sand), soil is at field capacity. Typically, at field capacity, air is in the macropores and water in micropores. Field capacity is viewed as the optimal condition for plant growth and microbial activity. At a potential of -1500 kPa, soil is at its permanent wilting point. At the permanent wilting point, soil water is held by solid particles as a "water film" that is retained too tightly to be taken up by plants. A tensiometer, electrical resistance block, neutron probes, or time-domain reflectometry (TDR) can be used to determine soil water potential. Tensiometers are limited to 0 to -85 kPa, electrical resistance blocks is limited to -90 to -1500 kPa, neutron probes is limited to 0 to -1500 kPa, and TDR is limited to 0 to -10,000 kPa.
See Also
Notes
- ↑ Taiz and Zeiger, Plant Physiology, Fourth Edition, Sinauer Associates, 2002
- ↑ Statkraft to build world's first osmotic power plant