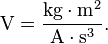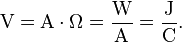Volt
| | |
 | |
| | |
| | |
| Unit system | SI derived unit |
| Unit of | Electric potential, electromotive force |
| Symbol | V |
| Named after | Alessandro Volta |
| In SI base units: | 1 V = 1 kg·m2·s-3·A-1 |
The volt (symbol: V) is the SI derived unit for electric potential (voltage), electric potential difference, and electromotive force.[1] The volt is named in honour of the Italian physicist Alessandro Volta (1745–1827), who invented the voltaic pile, possibly the first chemical battery.
Definition
A single volt is defined as the difference in electric potential between two points of a conducting wire when an electric current of one ampere dissipates one watt of power between those points.[2] It is also equal to the potential difference between two parallel, infinite planes spaced 1 meter apart that create an electric field of 1 newton per coulomb. Additionally, it is the potential difference between two points that will impart one joule of energy per coulomb of charge that passes through it. It can be expressed in terms of SI base units ( m, kg, s, and A) as:
It can also be expressed as amps×ohms (Ohm's law), power per unit current (Joule's law), or energy per unit charge:
Josephson junction definition
Between 1990 and 1997, the volt was calibrated using the Josephson effect for exact voltage-to-frequency conversion, combined with cesium-133 time reference, as decided by the 18th General Conference on Weights and Measures. The following value for the Josephson constant is used:
- K{J-90} = 2e/h = 0.4835979 GHz/µV,
where e is the elementary charge and h is the Planck constant.
This is typically used with an array of several thousand or tens of thousands of junctions, excited by microwave signals between 10 and 80 GHz (depending on the array design).[3] Empirically, several experiments have shown that the method is independent of device design, material, measurement setup, etc., and no correction terms are required in a practical implementation.[4]
Water flow analogy
In the water flow analogy sometimes used to explain electric circuits by comparing them to water-filled pipes, voltage (difference in electric potential) is likened to difference in water pressure.
The relationship between voltage and current is defined (in ohmic devices) by Ohm's Law.
Common voltages


Nominal voltages of familiar sources:
- Nerve cell resting potential: around −75 mV[5]
- Single-cell, rechargeable NiMH or NiCd battery: 1.2 V
- Mercury battery: 1.355 V
- Single-cell, non-rechargeable alkaline battery (e.g., AAA, AA, C and D cells): 1.5 V
- LiFePO4 rechargeable battery: 3.3 V
- Lithium polymer rechargeable battery: 3.75 V (see Rechargeable battery#Table of rechargeable battery technologies)
- Transistor-transistor logic/CMOS (TTL) power supply: 5 V
- USB: 5 V DC
- PP3 battery: 9 V
- Automobile electrical system: nominal 12 V, about 11.8 V discharged, 12.8 V charged, and 13.8–14.4 V while charging (vehicle running).
- Household mains electricity: 230 V RMS in Europe, Asia and Africa, 120 V RMS in North America, 100 V RMS in Japan (see List of countries with mains power plugs, voltages and frequencies)
- Trucks/lorries: 24 V DC
- Rapid transit third rail: 600–750 V (see List of current systems for electric rail traction)
- High-speed train overhead power lines: 25 kV RMS at 50 Hz, but see the list of current systems for electric rail traction and 25 kV at 60 Hz for exceptions.
- High-voltage electric power transmission lines: 110 kV RMS and up (1.15 MV RMS was the record as of 2005[citation needed])
- Lightning: Varies greatly, often around 100 MV.
Note: Where RMS (root mean square) is stated above, the peak voltage is  times greater than the RMS voltage for a sinusoidal signal centered around zero voltage.
times greater than the RMS voltage for a sinusoidal signal centered around zero voltage.
History

In 1800, as the result of a professional disagreement over the galvanic response advocated by Luigi Galvani, Alessandro Volta developed the so-called Voltaic pile, a forerunner of the battery, which produced a steady electric current. Volta had determined that the most effective pair of dissimilar metals to produce electricity is zinc and silver. In the 1880s, the International Electrical Congress, now the International Electrotechnical Commission (IEC), approved the volt as the unit for electromotive force. They made the volt equal to 108 cgs units of voltage, the cgs system at the time being the customary system of units in science. They chose such a ratio because the cgs unit of voltage is inconveniently small and one volt in this definition is approximately the emf of a Daniell cell, the standard source of voltage in the telegraph systems of the day.[6] At that time, the volt was defined as the potential difference [i.e., what is nowadays called the "voltage (difference)"] across a conductor when a current of one ampere dissipates one watt of power.
The international volt was defined in 1893 as 1/1.434 of the emf of a Clark cell. This definition was abandoned in 1908 in favor of a definition based on the international ohm and international ampere until the entire set of "reproducible units" was abandoned in 1948.
Prior to the development of the Josephson junction voltage standard, the volt was maintained in national laboratories using specially constructed batteries called standard cells. The United States used a design called the Weston cell from 1905 to 1972.
This SI unit is named after Alessandro Volta. As with every International System of Units (SI) unit whose name is derived from the proper name of a person, the first letter of its symbol is upper case (V). However, when an SI unit is spelled out in English, it should always begin with a lower case letter (volt), except in a situation where any word in that position would be capitalized, such as at the beginning of a sentence or in capitalized material such as a title. Note that "degree Celsius" conforms to this rule because the "d" is lowercase. —Based on The International System of Units, section 5.2.
See also
|
|
Notes and references
- ↑ "SI Brochure, Table 3 (Section 2.2.2)". BIPM. 2006. Retrieved 2007-07-29.
- ↑ BIPM SI Brochure: Appendix 1, p. 144
- ↑ Burroughs, Charles J.; Benz, Samuel P. (1999-06-01), "1 Volt DC Programmable Josephson Voltage Standard", IEEE transactions on applied superconductivity 9 (3): 4145–4149, ISSN 1051-8223
- ↑ Keller, Mark W (2008-01-18), "Current status of the quantum metrology triangle", Metrologia 45 (1): 102–109, Bibcode:2008Metro..45..102K, doi:10.1088/0026-1394/45/1/014, ISSN 0026-1394, "Theoretically, there are no current predictions for any correction terms. Empirically, several experiments have shown that KJ and RK are independent of device design, material, measurement setup, etc. This demonstration of universality is consistent with the exactness of the relations, but does not prove it outright."
- ↑ Bullock, Orkand, and Grinnell, pp. 150–151; Junge, pp. 89–90; Schmidt-Nielsen, p. 484
- ↑ Hamer, Walter J. (January 15, 1965). Standard Cells: Their Construction, Maintenance, and Characteristics. National Bureau of Standards Monograph #84. US National Bureau of Standards.
External links
| Look up volt in Wiktionary, the free dictionary. |
| ||||||||||||||||||||