Voglibose
 | |
|---|---|
| Systematic (IUPAC) name | |
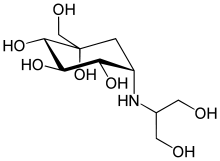
| (1S,2S,3R,4S,5S)-5-(1,3-dihydroxypropan-2-ylamino)-1-(hydroxymethyl)cyclohexane-1,2,3,4-tetraol | |
| Clinical data | |
| AHFS/Drugs.com | International Drug Names |
| Legal status | ? |
| Identifiers | |
| CAS number | 83480-29-9 |
| ATC code | A10BF03 |
| PubChem | CID 444020 |
| DrugBank | DB04878 |
| ChemSpider | 392046 |
| UNII | S77P977AG8 |
| KEGG | D01665 |
| ChEMBL | CHEMBL476960 |
| Chemical data | |
| Formula | C10H21NO7 |
| Mol. mass | 267.28 g/mol |
| SMILES
| |
| |
| | |
Voglibose (INN and USAN, trade name Voglib, marketed by Mascot Health Series) is an alpha-glucosidase inhibitor used for lowering post-prandial blood glucose levels in people with diabetes mellitus. Voglibose delays the absorption of glucose thereby reduces the risk of macrovascular complications. Voglibose is a research product of Takeda Pharma, a Japan-based company.
Postprandial hyperglycemia (PPHG) is primarily due to first phase insulin secretion. Alpha glucosidase inhibitors delay glucose absorption at the intestine level and thereby prevent sudden surge of glucose after a meal.
There are three drugs which belong to this class, acarbose, miglitol and voglibose, of which voglibose is the newest. Voglibose scores over both acarbose and miglitol in terms of side effect profile. But acarbose has an edge over voglibose in terms of efficacy (FPG, PPHG, HBA1c).
There are several trials supporting the use of voglibose in the management of PPHG. Also, it has been established that it is PPHG, not FPG, which is the marker of cardiovascular disorders associated with diabetes. So, controlling PPHG is imperative and voglibose is indicated for the management of PPHG.
External links
- Ranbaxy announcement (Bad link)
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||