Virial expansion
The classical virial expansion expresses the pressure of a many-particle system in equilibrium as a power series in the density.
The virial expansion, introduced in 1901 by Heike Kamerlingh Onnes, is a generalization of the ideal gas law. He wrote that for a gas containing  atoms
or molecules,
atoms
or molecules,
where  is the pressure,
is the pressure,  is the Boltzmann constant,
is the Boltzmann constant,  is the absolute temperature, and
is the absolute temperature, and
 is the number density of the
gas.
Note that for a gas containing a fraction
is the number density of the
gas.
Note that for a gas containing a fraction  of
of 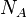 (Avogadro's number) molecules, truncation of the virial expansion after the
first term leads to
(Avogadro's number) molecules, truncation of the virial expansion after the
first term leads to 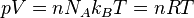 , which is the ideal gas law.
, which is the ideal gas law.
Writing 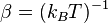 , the virial expansion can be written in closed form as
, the virial expansion can be written in closed form as
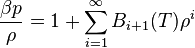 .
.
The virial coefficients  are characteristic of the interactions between the particles in the system and in general depend on the temperature
are characteristic of the interactions between the particles in the system and in general depend on the temperature  .
.
