Variegatic acid
| Variegatic acid | ||
|---|---|---|
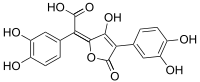 | ||
| IUPAC name α-[4-(3,4-Dihydroxyphenyl)-3-hydroxy-5-oxofuran-2(5H)-ylidene]-3,4-dihydroxybenzeneacetic acid | ||
| Other names 3,3',4,4'-tetrahydroxy pulvinic acid | ||
| Identifiers | ||
| CAS number | 20988-30-1 | |
| Jmol-3D images | Image 1 | |
| ||
| ||
| Properties | ||
| Molecular formula | C18H12O9 | |
| Molar mass | 372.28 g mol−1 | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Variegatic acid (3,3',4,4'-tetrahydroxypulvinic acid) is a chemical. An orange pigment, it is responsible for the bluing reaction seen in many bolete mushrooms when they are injured. When mushroom tissue containing variegatic acid is exposed to air, the chemical is enzymatically oxidized to blue chinonmethid anions.[1]
It was first isolated from Suillus variegatus.[2] It has strong antioxidant properties,[3][4] and a nonspecific inhibitory effect on cytochrome P450 enzymes.[5] A total synthesis was reported in 2001 that uses a Suzuki cross coupling reaction.[6]
See also
References
- ↑ Velíšek J, Cejpek K. (2011). "Pigments of higher fungi: A review" (PDF). Czech Journal of Food Science 29 (2): 87–102.
- ↑ Edwards RL, Elsworthy GC. (1967). "Variegatic acid, a new tetronic acid responsible for the blueing reaction in the fungus Suillus (Boletus) variegatus (Swartz ex Fr.)". Chemical Communications (London) (8): 373b–374. doi:10.1039/C1967000373B.
- ↑ Kasuga A, Aoyagi Y, Sugahara T. (1995). "Antioxidant activity of fungus Suillus bovinus (L: Fr.) O. Kuntze". Journal of Food Science 60 (5): 1113–85. doi:10.1111/j.1365-2621.1995.tb06304.x.
- ↑ Vidovic SS, Mujic IO, Zekovic ZP, Lepojevic ZD, Tumbas VT, Mujic AI. (2010). "Antioxidant properties of selected Boletus mushrooms". Food Biophysics 5 (1): 49–58. doi:10.1007/s11483-009-9143-6.
- ↑ Huang YT, Onose J, Abe N, Yoshikawa K. (2009). "In vitro inhibitory effects of pulvinic acid derivatives isolated from Chinese edible mushrooms, Boletus calopus and Suillus bovinus, on cytochrome P450 activity". Bioscience, Biotechnology, and Biochemistry 73 (4): 855–60. doi:10.1271/bbb.80759. PMID 19352038.

- ↑ Ahmed Z, Langer P. (2005). "Synthesis of natural pulvinic acids based on a '[3+2] cyclization-Suzuki cross-coupling' strategy". Tetrahedron 61 (8): 2055–63. doi:10.1016/j.tet.2004.12.048.