Uridine diphosphate N-acetylglucosamine
| Uridine diphosphate N-acetylglucosamine | ||
|---|---|---|
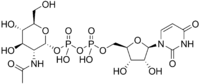 | ||
| IUPAC name [(2R,3R,4R,5S,6R)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl] [[(2R,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] hydrogen phosphate | ||
| Other names UDP-N-acetylglucosamine; UDP-GlcNAc | ||
| Identifiers | ||
| CAS number | 7277-98-7 | |
| PubChem | 445675 | |
| Jmol-3D images | {{#if:CC(=O)N[C@@H]1[C@H]([C@@H]([C@H](O[C@@H]1OP(=O)(O)OP(=O)(O)OC[C@@H]2[C@H]([C@H]([C@@H](O2)N3C=CC(=O)NC3=O)O)O)CO)O)O|Image 1 | |
| ||
| Properties | ||
| Molecular formula | C17H27N3O17P2 | |
| Molar mass | 607.35 g mol−1 | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Uridine diphosphate N-acetylglucosamine or UDP-GlcNAc is a nucleotide sugar and a coenzyme in metabolism. It is used by glycosyltransferases to transfer N-acetylglucosamine residues to substrates. D-Glucosamine is made naturally in the form of glucosamine-6-phosphate, and is the biochemical precursor of all nitrogen-containing sugars.[1] To be specific, glucosamine-6-phosphate is synthesized from fructose 6-phosphate and glutamine[2] as the first step of the hexosamine biosynthesis pathway.[3] The end-product of this pathway is UDP-GlcNAc, which is then used for making glycosaminoglycans, proteoglycans, and glycolipids.[4]
UDP-GlcNAc is extensively involved in intracellular signaling as a substrate for O-linked N-acetylglucosamine transferases (OGTs) in a wide range of species. It is also involved in nuclear pore formation and nuclear signalling. OGTs and OG-ases play an important role in the structure of the cytoskeleton. In mammals, there is enrichment of OGT transcripts in the pancreas beta-cells, and UDP-GlcNAc is thought to be part of the glucose sensing mechanism. There is also evidence that it plays a part in insulin sensitivity in other cells. In plants, it is involved in the control of gibberellin production.[5]
Clostridium novyi type A alpha-toxin is an O-linked N-actetylglucosamine transferase acting on Rho proteins and causing the collapse of the cytoskeleton.
References
- ↑ Roseman, S (2001). "Reflections on glycobiology". The Journal of Biological Chemistry 276 (45): 41527–42. doi:10.1074/jbc.R100053200. PMID 11553646.
- ↑ Sudhamoy Ghosh; Blumenthal, HJ; Davidson, E; Roseman, S (1960-05-01). "Glucosamine Metabolism". Journal of Biological Chemistry 235 (5): 1265. PMID 13827775.
- ↑ International Union of Biochemistry and Molecular Biology
- ↑ Milewski S, Gabriel I, Olchowy J (2006). "Enzymes of UDP-GlcNAc biosynthesis in yeast". Yeast 23 (1): 1–14. doi:10.1002/yea.1337. PMID 16408321.
- ↑ http://www.fasebj.org/cgi/content/full/15/11/1865 Hanover J. A.: Glycan-dependent signaling: O-linked N-acetylglucosamine. The FASEB Journal. 2001;15:1865-1876.
| ||||||||