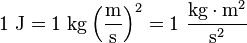Units of energy
Because energy is defined via work, the SI unit for energy is the same as the unit of work – the joule (J), named in honor of James Prescott Joule and his experiments on the mechanical equivalent of heat. In slightly more fundamental terms, 1 joule is equal to 1 newton-metre and, in terms of SI base units
An energy unit that is used in atomic physics, particle physics and high energy physics is the electronvolt (eV). One eV is equivalent to 1.60217653×10−19 J. In spectroscopy the unit cm−1 = 0.000123986 eV is used to represent energy since energy is inversely proportional to wavelength from the equation 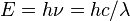 .
.
In discussions of energy production and consumption, the units barrel of oil equivalent and ton of oil equivalent are often used.
When discussing amounts of energy released in explosions or bolide impact events, the TNT equivalent unit is often used. 1 ton of TNT equivalent is equal to 4.2 × 109 joules. Therefore, 1 kt TNT is 4.2 × 1012 joules, and 1 Mt TNT is 4.2 × 1015 joules.
Note that torque, the "rotational force" or "angular force" which causes a change in rotational motion is typically expressed in newton-metres. This is not a simple coincidence: a torque of 1 newton-metre applied on 1 radian requires exactly 1 newton-metre = 1 joule of energy.
Other units of energy
In cgs units, one erg is 1 g cm2 s−2, equal to 1.0×10−7 J.
US units
The imperial/U.S. units for both energy and work include the foot-pound force (1.3558 J), the British thermal unit (Btu) which has various values in the region of 1055 J, and the horsepower-hour (2.6845 MJ).
Electricity
The energy unit used for everyday electricity, particularly for utility bills, is the kilowatt-hour (kWh), and one kWh is equivalent to 3.6×106 J (3600 kJ or 3.6 MJ). Electricity usage is often given in units of kilowatt-hours per year (kWh/yr). This is actually a measurement of average power consumption, i.e., the average rate at which energy is transferred.
Natural gas
Natural gas in the US is sold in Therms or 100 cubic feet. One Therm is equal to about 105.5 megajoules. In the rest of the world, natural gas is sold in gigajoules.
Food industry
The calorie equals the amount of thermal energy necessary to raise the temperature of one gram of water by 1 Celsius degree, at a pressure of 1 atm. For thermochemistry a calorie of 4.184 J is used, but other calories have also been defined, such as the International Steam Table calorie of 4.1868 J. Food energy is measured in large calories or kilocalories, often simply written capitalized as "Calories" (= 103 calories).
Atom physics and chemistry
In physics and chemistry, it is still common to measure energy on the atomic scale in the non-SI, but convenient, units electronvolts (eV). The Hartree (the atomic unit of energy) is commonly used in calculations. Historically Rydberg units have been used.
Spectroscopy
In spectroscopy and related fields it is common to measure energy levels in units of reciprocal centimetres. These units (cm−1) are strictly speaking not energy units but units proportional to energies, with 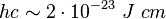 being the proportionality constant.[1]
being the proportionality constant.[1]
Explosions
A gram of TNT releases 980–1100 calories upon explosion. To define the tonne of TNT, this was arbitrarily standardized by letting 1000 thermochemical calories = 1 gram TNT = 4184 J (exactly).[2]
See also
- Energy consumption
- Conversion of units of temperature
- Conversion of units#Energy, work, or amount of heat
- Orders of magnitude (energy)
References
- ↑ "CCCBDB What's a cm-1?". Cccbdb.nist.gov. Retrieved 2013-11-13.
- ↑ NIST Guide for the Use of the International System of Units (SI): Appendix B8—Factors for Units Listed Alphabetically
| ||||||||||||||