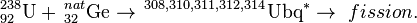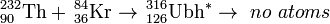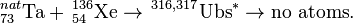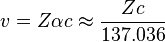Superactinide
| Superactinides in the periodic table | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
A superactinide is any one of 32 hypothetical chemical elements from atomic numbers 121 (unbiunium) through 153 (unpenttrium), at which the 5g and 6f electron shells are filled up. They may be referred to using IUPAC systematic element names. None of these elements have been synthesized,[note 1] and it is possible that none have isotopes with stable enough nuclei to receive significant attention in the near future. Synthesis has only been attempted for elements 122, 124, 126, and 127. It is also probable that, due to drip instabilities, only the lighter superactinides are physically possible and the periodic table may end soon after the island of stability expected to be centered at element 126.[2] The theoretical existence of the series was proposed by Glenn T. Seaborg, winner of the 1951 Nobel Prize in Chemistry.
If it were possible to produce sufficient quantities of these elements that would allow the study of their chemistry, these elements might well behave very differently from those of previous periods. This is because their electronic configurations may be altered by quantum and relativistic effects, as the energy levels of the 5g, 6f and 7d orbitals are so close to each other that they may well exchange electrons with each other.[3] This would result in a large number of elements in the superactinide series that would have extremely similar chemical properties that would be quite unrelated to elements of lower atomic numbers.[2]
History
There are currently seven periods in the periodic table of chemical elements, culminating with atomic number 118. If further elements with higher atomic numbers than this are discovered, they will be placed in additional periods, laid out (as with the existing periods) to illustrate periodically recurring trends in the properties of the elements concerned. Any additional periods are expected to contain a larger number of elements than the seventh period, as they are calculated to have an additional so-called g-block, containing 18 elements with partially filled g-orbitals in each period. An eight-period table containing this block was suggested by Glenn T. Seaborg in 1969.[4][5] No elements in this region have been synthesized or discovered in nature.[note 2] While Seaborg's version of the extended period had the heavier elements following the pattern set by lighter elements, other models do not. Pekka Pyykkö, for example, used computer modeling to calculate the positions of elements up to Z = 172, and found that several were displaced from the Madelung rule.[6]
Expected properties
The first element of the g-block may have atomic number 121, and thus would have the systematic name unbiunium. Elements in this region are likely to be highly unstable with respect to radioactive decay, and have extremely short half lives, although element 126 is hypothesized to be within an island of stability that is resistant to fission but not to alpha decay. It is not clear how many elements beyond the expected island of stability are physically possible, or even if the superactindes are complete.
According to the orbital approximation in quantum mechanical descriptions of atomic structure, the g-block would correspond to elements with partially filled g-orbitals. However, spin-orbit coupling effects reduce the validity of the orbital approximation substantially for elements of high atomic numbers.
Elements
If superheavy elements continue to follow the Aufbau principle, the superactinide series contains the following elements:
| Superactinides in the extended periodic table | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 121 Ubu |
122 Ubb |
123 Ubt |
124 Ubq |
125 Ubp |
126 Ubh |
127 Ubs |
128 Ubo |
129 Ube |
130 Utn |
131 Utu |
132 Utb |
133 Utt |
134 Utq |
135 Utp |
136 Uth |
137 Uts |
138 Uto |
139 Ute |
140 Uqn |
141 Uqu |
142 Uqb |
143 Uqt |
144 Uqq |
145 Uqp |
146 Uqh |
147 Uqs |
148 Uqo |
149 Uqe |
150 Upn |
151 Upu |
152 Upb |
153 Upt | ||||||||||||||||||
| g-block | f-block | d-block | ||||||||||||||||||||||||||||||||||||||||||||||||
If the Pyykkö model is correct, the superactinide series contains the following elements instead:[7]
| Legend | |||||||||||||
| |||||||||||||
All of these hypothetical undiscovered elements are named by the International Union of Pure and Applied Chemistry (IUPAC) systematic element name standard which creates a generic name for use until the element has been discovered, confirmed, and an official name approved.
g-block superactinides
Attempts at synthesis

The only elements in this region of the periodic table that have had attempts to synthesise them are elements 122, 124, 126, and 127.
The first attempt to synthesize unbibium was performed in 1972 by Flerov et al. at JINR, using the hot fusion reaction:
No atoms were detected and a yield limit of 5 mb (5,000,000 pb) was measured. Current results (see flerovium) have shown that the sensitivity of this experiment was too low by at least 6 orders of magnitude.
In 2000, the Gesellschaft für Schwerionenforschung performed a very similar experiment with much higher sensitivity:
These results indicate that the synthesis of such heavier elements remains a significant challenge and further improvements of beam intensity and experimental efficiency is required. The sensitivity should be increased to 1 fb.
Several experiments have been performed between 2000 and 2004 at the Flerov laboratory of Nuclear Reactions studying the fission characteristics of the compound nucleus 306Ubb. Two nuclear reactions have been used, namely 248Cm+58Fe and 242Pu+64Ni. The results have revealed how nuclei such as this fission predominantly by expelling closed shell nuclei such as 132Sn (Z=50, N=82). It was also found that the yield for the fusion-fission pathway was similar between 48Ca and 58Fe projectiles, indicating a possible future use of 58Fe projectiles in superheavy element formation.[8]
On April 24, 2008, a group led by Amnon Marinov at the Hebrew University of Jerusalem claimed to have found single atoms of unbibium in naturally occurring thorium deposits at an abundance of between 10−11 and 10−12, relative to thorium.[9] The claim of Marinov et al. was criticized by a part of the scientific community, and Marinov says he has submitted the article to the journals Nature and Nature Physics but both turned it down without sending it for peer review.[1]
A criticism of the technique, previously used in purportedly identifying lighter thorium isotopes by mass spectrometry,[10][11] was published in Physical Review C in 2008.[12] A rebuttal by the Marinov group was published in Physical Review C after the published comment.[13]
A repeat of the thorium-experiment using the superior method of Accelerator Mass Spectrometry (AMS) failed to confirm the results, despite a 100-fold better sensitivity.[14] This result throws considerable doubt on the results of the Marinov collaboration with regards to their claims of long-lived isotopes of thorium, roentgenium and unbibium.
In a series of experiments, scientists at GANIL have attempted to measure the direct and delayed fission of compound nuclei of elements with Z=114, 120, and 124 in order to probe shell effects in this region and to pinpoint the next spherical proton shell. In 2006, with full results published in 2008, the team provided results from a reaction involving the bombardment of a natural germanium target with uranium ions:
The team reported that they had been able to identify compound nuclei fissioning with half-lives > 10−18 s. Although very short, the ability to measure such decays indicated a strong shell effect at Z=124. A similar phenomenon was found for Z=120 but not for Z=114.[15]

The first attempt to synthesize unbihexium was performed in 1971 by Bimbot et al. using the hot fusion reaction:
A high energy alpha particle was observed and taken as possible evidence for the synthesis of unbihexium. Recent research suggests that this is highly unlikely as the sensitivity of experiments performed in 1971 would have been several orders of magnitude too low according to current understanding. To date, no other attempt has been made to synthesize unbihexium.
Unbiseptium has had one failed attempt at synthesis in 1978 at the Darmstadt UNILAC accelerator by bombarding a natural tantalum target with xenon ions. No atoms were detected.[16]
Element 137
Untriseptium, element 137, is sometimes called feynmanium (symbol Fy) because Richard Feynman noted[17] that a simplistic interpretation of the relativistic Dirac equation runs into problems with electron orbitals at Z > 1/α = 137, suggesting that neutral atoms cannot exist beyond untriseptium, and that a periodic table of elements based on electron orbitals therefore breaks down at this point. However, a more rigorous analysis calculates the limit to be Z ≈ 173, indicating that unsepttrium (element 173) is in fact the last possible neutral atom.
Bohr model breakdown
The Bohr model exhibits difficulty for atoms with atomic number greater than 137, for the speed of an electron in a 1s electron orbital, v, is given by
where Z is the atomic number, and α is the fine structure constant, a measure of the strength of electromagnetic interactions.[18] Under this approximation, any element with an atomic number greater than 137 would require 1s electrons to be traveling faster than c, the speed of light. Hence the non-relativistic Bohr model is clearly inaccurate when applied to such an element.
The Dirac equation
The relativistic Dirac equation also has problems for Z > 137, for the ground state energy is
where m is the rest mass of the electron. For Z > 137, the wave function of the Dirac ground state is oscillatory, rather than bound, and there is no gap between the positive and negative energy spectra, as in the Klein paradox.[19]
More accurate calculations including the effects of the finite size of the nucleus indicate that the binding energy first exceeds 2mc2 for Z > Zcr ≈ 173. ForZ > Zcr, if the innermost orbital is not filled, the electric field of the nucleus will pull an electron out of the vacuum, resulting in the spontaneous emission of a positron.[20]
f-block and d-block superactinides
The relativistic and quantum effects for the electron clouds of the f-block elements are expected to be even greater than those for the g-block elements, because these elements have higher atomic number. If these elements could actually be observed, they would likely be observed to have similar chemical properties, but the effect of the closeness of the 5g and 6f (and possibly also the 7d and 8p) subshells is unclear and difficult to predict because of the relativistic and quantum effects. These orbitals, being so close in energy, may fill together all at the same time, resulting in a series of very similar elements with many barely distinguishable oxidation states. The basis of periodic trends based on electron configurations may thus no longer hold.[2]
The existence of such atoms is probably theoretically possible as the upper limit for atomic number is likely Z = 173 due to the light-speed limit,[21] after which assigning electron shells would be nonsensical and elements would only be able to exist as ions, but it is not clear if our technology will ever be enough to synthesise them.
Although element 153 would likely be taken to be the last superactinide based on previous periods, the electron configurations for the d-block and p-block period 8 elements would likely be nothing more than mathematical extrapolation because of the extreme quantum and relativistic effects the electron clouds will experience. In the unlikely case that their chemical properties may eventually be studied, it is likely that all existing classifications will be inadequate to describe them. Due to the breakdown of periodic trends expected in this region due to the closeness of energy of the 5g, 6f, 7d and 8p orbitals and other relativistic effects, it seems likely that the properties and placement in the periodic table of these elements may be of only formal significance.[2]
Related substances
Lanthanides and actinides
Eka-superactinides
| Eka-superactinides in the periodic table | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Related to the superactinides are the eka-superactinides, one row further down in the periodic table, which are elements 171 through 203.[21]
See also
Notes
- ↑ The heaviest element that has been synthesized to date is ununoctium with atomic number 118, which is the last period 7 element.
- ↑ Element 122 was claimed to exist naturally in April 2008 by Amnon Marinov, but this claim was widely believed to be erroneous.[1]
Bibliography
- J. Huheey: Anorganische Chemie, 2. Auflage, 1995
References
- ↑ 1.0 1.1 Royal Society of Chemistry, "Heaviest element claim criticised", Chemical World.
- ↑ 2.0 2.1 2.2 2.3 Seaborg (c. 2006). "transuranium element (chemical element)". Encyclopædia Britannica. Retrieved 2010-03-16.
- ↑ Waber, J. T. (1969). "SCF Dirac–Slater Calculations of the Translawrencium Elements". The Journal of Chemical Physics 51 (2): 664–661. Bibcode:1969JChPh..51..664W. doi:10.1063/1.1672054.
- ↑ Seaborg, Glenn (August 26, 1996). "An Early History of LBNL".
- ↑ Frazier, K. (1978). "Superheavy Elements". Science News 113 (15): 236–238. doi:10.2307/3963006. JSTOR 3963006.
- ↑ "Extended elements: new periodic table". 2010.
- ↑ Pyykkö, Pekka (2011). "A suggested periodic table up to Z≤ 172, based on Dirac–Fock calculations on atoms and ions". Physical Chemistry Chemical Physics 13 (1): 161–8. Bibcode:2011PCCP...13..161P. doi:10.1039/c0cp01575j. PMID 20967377.
- ↑ see Flerov lab annual reports 2000–2004 inclusive http://www1.jinr.ru/Reports/Reports_eng_arh.html
- ↑ Marinov, A.; Rodushkin, I.; Kolb, D.; Pape, A.; Kashiv, Y.; Brandt, R.; Gentry, R. V.; Miller, H. W. (2008). "Evidence for a long-lived superheavy nucleus with atomic mass number A=292 and atomic number Z=~122 in natural Th". International Journal of Modern Physics E 19: 131. arXiv:0804.3869. Bibcode:2010IJMPE..19..131M. doi:10.1142/S0218301310014662.
- ↑ A. Marinov; I. Rodushkin; Y. Kashiv; L. Halicz; I. Segal; A. Pape; R. V. Gentry; H. W. Miller; D. Kolb; R. Brandt (2007). "Existence of long-lived isomeric states in naturally-occurring neutron-deficient Th isotopes". Phys. Rev. C 76 (2): 021303(R). arXiv:nucl-ex/0605008. Bibcode:2007PhRvC..76b1303M. doi:10.1103/PhysRevC.76.021303.
- ↑ Marinov, A.; Rodushkin, I.; Kashiv, Y.; Halicz, L.; Segal, I.; Pape, A.; Gentry, R.; Miller, H.; Kolb, D.; Brandt, R. (2007). "Existence of long-lived isomeric states in naturally-occurring neutron-deficient Th isotopes". Physical Review C 76 (2): 021303. arXiv:nucl-ex/0605008. Bibcode:2007PhRvC..76b1303M. doi:10.1103/PhysRevC.76.021303.
- ↑ R. C. Barber; J. R. De Laeter (2009). "Comment on "Existence of long-lived isomeric states in naturally-occurring neutron-deficient Th isotopes"". Phys. Rev. C 79 (4): 049801. Bibcode:2009PhRvC..79d9801B. doi:10.1103/PhysRevC.79.049801.
- ↑ A. Marinov; I. Rodushkin; Y. Kashiv; L. Halicz; I. Segal; A. Pape; R. V. Gentry; H. W. Miller; D. Kolb; R. Brandt (2009). "Reply to "Comment on 'Existence of long-lived isomeric states in naturally-occurring neutron-deficient Th isotopes'"". Phys. Rev. C 79 (4): 049802. Bibcode:2009PhRvC..79d9802M. doi:10.1103/PhysRevC.79.049802.
- ↑ J. Lachner; I. Dillmann; T. Faestermann; G. Korschinek; M. Poutivtsev; G. Rugel (2008). "Search for long-lived isomeric states in neutron-deficient thorium isotopes". Phys. Rev. C 78 (6): 064313. arXiv:0907.0126. Bibcode:2008PhRvC..78f4313L. doi:10.1103/PhysRevC.78.064313.
- ↑ M. Morjean; J. L. Charvet; A. Chbini; M. Chevallier; C. Cohen; D. Dauvergne; R. Dayras; A. Drouart; J. D. Frankland; D. Jacquet; R. Kirsch; M. Laget; P. Lautesse; A. L'hoir; A. Marchix; L. Naplas; M. Parlog; C. Ray; C. Schmitt; C. Stodel; L. Tassan-Got; C. Volant (2007). "Direct experimental evidence for very long fission times of super-heavy elements". European Physical Journal D.
- ↑ Emsley, John (2011). Nature's Building Blocks: An A-Z Guide to the Elements (New ed.). New York, NY: Oxford University Press. p. 593. ISBN 978-0-19-960563-7.
- ↑ G. Elert. "Atomic Models". The Physics Hypertextbook. Retrieved 2009-10-09.
- ↑ See for example R. Eisberg, R. Resnick (1985). Quantum Physics of Atoms, Molecules, Solids, Nuclei and Particles. Wiley.
- ↑ J.D. Bjorken, S.D. Drell (1964). Relativistic Quantum Mechanics. McGraw-Hill.
- ↑ W. Greiner, S. Schramm (2008). American Journal of Physics 76. p. 509., and references therein.
- ↑ 21.0 21.1 Walter Greiner and Stefan Schramm (2008). "Resource Letter QEDV-1: The QED vacuum". American Journal of Physics 76 (6): 509. Bibcode:2008AmJPh..76..509G. doi:10.1119/1.2820395., and references therein.
| Extended periodic table | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |||||||||||||||||||||||||||||||
| 1 | H | He | ||||||||||||||||||||||||||||||||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||||||||
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||||||||
| 6 | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||||||||
| 7 | Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | 113 | Fl | 115 | Lv | 117 | 118 | ||||||||||||||||
| 8 | 119 | 120 | * | 141 | 142 | 143 | 144 | 145 | 146 | 147 | 148 | 149 | 150 | 151 | 152 | 153 | 154 | 155 | 156 | 157 | 158 | 159 | 160 | 161 | 162 | 163 | 164 | |||||||||||||||||||||
| 9 | 165 | 166 | 167 | 168 | 169 | 170 | 171 | 172 | ||||||||||||||||||||||||||||||||||||||||
| 10 | ** | |||||||||||||||||||||||||||||||||||||||||||||||
| * | 121 | 122 | 123 | 124 | 125 | 126 | 127 | 128 | 129 | 130 | 131 | 132 | 133 | 134 | 135 | 136 | 137 | 138 | 139 | 140 | ||||||||||||||||||||||||||||
| ** |
| |||||||||||||||||||||||||||||||||||||||||||||||
|
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Large version | ||||||||||||||||||||||||||||||||||||||||||||||||