Tyramine
| Tyramine | |
|---|---|
 | |
 | |
| IUPAC name 4-(2-aminoethyl)phenol | |
| Identifiers | |
| CAS number | 51-67-2 |
| PubChem | 5610 |
| ChemSpider | 5408 |
| UNII | X8ZC7V0OX3 |
| KEGG | C00483 |
| MeSH | Tyramine |
| ChEBI | CHEBI:15760 |
| ChEMBL | CHEMBL11608 |
| Jmol-3D images | Image 1 |
| |
| |
| Properties | |
| Molecular formula | C8H11NO |
| Molar mass | 137.179 g/mol[1] |
| Appearance | colorless solid |
| Density | 1.20 g/cm 3[2] |
| Melting point | 164-165°C [3] |
| Boiling point | 205-207°C at 25 mm Hg; 166°C at 2 mm Hg [3] |
| Solubility in water | 1 g in 95 mL at 15°C [3] |
| Acidity (pKa) | 9.74 (OH); 10.52 (NH3+) [4] |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Tyramine (4-hydroxyphenethylamine; para-tyramine, mydrial or uteramin) is a naturally occurring monoamine compound and trace amine derived from the amino acid tyrosine.[1] Tyramine acts as a catecholamine releasing agent. Notably, however, it is unable to cross the blood-brain barrier, resulting in only nonpsychoactive peripheral sympathomimetic effects. A hypertensive crisis can result from ingestion of tyramine-rich foods in conjunction with monoamine oxidase inhibitors (MAOIs).
Occurrence
Tyramine occurs widely in plants[5] and animals, and is metabolized by the enzyme monoamine oxidase. In foods, it is often produced by the decarboxylation of tyrosine during fermentation or decay. Foods containing considerable amounts of tyramine include meats that are potentially spoiled or pickled, aged, smoked, fermented, or marinated (some fish, poultry, and beef); most pork (except cured ham); chocolate; alcoholic beverages; and fermented foods, such as most cheeses (except ricotta, cottage, cream and Neufchâtel cheeses), sour cream, yogurt, shrimp paste, soy sauce, soybean condiments, teriyaki sauce, tempeh, miso soup, sauerkraut, kimchi, broad (fava) beans, green bean pods, Italian flat (Romano) beans, snow peas, avocados, bananas, pineapple, eggplants, figs, red plums, raspberries, peanuts, Brazil nuts, coconuts, processed meat, yeast, and an array of cacti.
Physical effects and pharmacology
Tyramine is physiologically metabolized by MAOA. In humans, if monoamine metabolism is compromised by the use of monoamine oxidase inhibitors (MAOIs) and foods high in tyramine are ingested, a hypertensive crisis can result, as tyramine can cause the release of stored monoamines, such as dopamine, norepinephrine and epinephrine. The first signs of this were discovered by a neurologist who noticed his wife, who at the time was on MAOI medication, had severe headaches when eating cheese.[6] For this reason, the crisis is still called the "cheese effect" or "cheese crisis", though other foods can cause the same problem.[7]:30-31 Most processed cheeses do not contain enough tyramine to cause hypertensive effects, although some aged cheeses (such as Stilton) do.[8][9]
A large dietary intake of tyramine (or a dietary intake of tyramine while taking MAO inhibitors) can cause the tyramine pressor response, which is defined as an increase in systolic blood pressure of 30 mmHg or more. The displacement of norepinephrine (noradrenaline) from neuronal storage vesicles by acute tyramine ingestion is thought to cause the vasoconstriction and increased heart rate and blood pressure of the pressor response. In severe cases, adrenergic crisis can occur.[medical citation needed]
However, if one has had repeated exposure to tyramine, there is a decreased pressor response; tyramine is degraded to octopamine, which is subsequently packaged in synaptic vesicles with norepinephrine (noradrenaline). Therefore, after repeated tyramine exposure, these vesicles contain an increased amount of octopamine and a relatively reduced amount of norepinephrine. When these vesicles are secreted upon tyramine ingestion, there is a decreased pressor response, as less norepinephrine is secreted into the synapse, and octopamine does not activate alpha or beta adrenergic receptors. [medical citation needed]
When using a MAO inhibitor (MAOI), the intake of approximately 10 to 25 mg of tyramine is required for a severe reaction compared to 6 to 10 mg for a mild reaction.[medical citation needed]
The possibility that tyramine acts directly as a neurotransmitter was revealed by the discovery of a G protein-coupled receptor with high affinity for tyramine, called TAAR1. The TAAR1 receptor is found in the brain, as well as peripheral tissues, including the kidneys. The existence of a receptor with high affinity for tyramine supports the hypothesis that tyramine may also act directly to affect blood pressure regulation.[medical citation needed]
Dietary tyramine intake has also been associated with migraine in select populations, leading many sufferers to restrict foods high in tyramine.[10] Reports on the tyramine-migraine link have been both affirmed and denied. At the Department of Neurology and EEG at The London Hospital, a double-blind study has shown no significant relationship between tyramine ingestion and migraines.[11] The EEG changes observed, however, do support tyramine as playing a role on the central nervous system in some subjects. A 2007 review published in Neurological Sciences[12] presented data showing migraine and cluster headaches are characterised by an increase of circulating neurotransmitters and neuromodulators (including tyramine, octopamine and synephrine) in the hypothalamus, amygdala and dopaminergic system.
Biosynthesis
Biochemically, tyramine is produced by the decarboxylation of tyrosine via the action of the enzyme tyrosine decarboxylase.[13] Tyramine can, in turn, be converted to methylated alkaloid derivatives N-methyltyramine, N,N-dimethyltyramine (hordenine), and N,N,N-trimethyltyramine (candicine).
-

Tyramine
-

N-Methyltyramine
-

N,N-Dimethyltyramine (hordenine)
-

N,N,N-Trimethyltyramine (candicine)
Chemistry
In the laboratory, tyramine can be synthesized in various ways, in particular by the decarboxylation of tyrosine.[14][15][16]
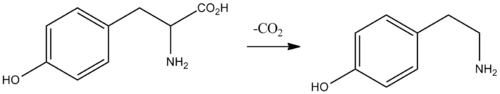
See also
- Trace amines
- Octopamine
- Phenethylamine
- N-methyltyramine
- Hordenine
- Candicine
References
- ↑ 1.0 1.1 PubChem
- ↑ A. M. Andersen (1977). "The crystal and molecular structure of tyramine hydrochloride." Acta Chem. Scandinavica B 31 162-166.
- ↑ 3.0 3.1 3.2 The Merck Index, 10th Ed. (1983), p.1405, Rahway: Merck & Co.
- ↑ Kappe, T. (1965). Journal of Medicinal Chemistry 8: 368–374
- ↑ T. A. Smith (1977) Phytochem. 16 9-18.
- ↑ Sathyanarayana Rao TS and Vikram K. Yeragani VK (2009) Hypertensive crisis and cheese Indian J Psychiatry. 51(1): 65–66.
- ↑ E. Siobhan Mitchell Antidepressants, chapter in Drugs, the Straight Facts, edited by David J. Triggle. 2004, Chelsea House Publishers
- ↑ Stahl SM, Felker A (2008). "Monoamine oxidase inhibitors: a modern guide to an unrequited class of antidepressants". Cns Spectrums 13 (10): 855–870. PMID 18955941.
- ↑ 's%20Instructions%20Patients/pdf/Pg570.pdf Tyramine-restricted Diet 1998, W.B. Saunders Company.
- ↑ Millichap, J. Gordon (Summer 2002). Noha News. XXVII: 3–6 http://www.nutrition4health.org/nohanews/NNS02DietMigraineHeadaches.htm
|url=missing title (help) - ↑ Moffett, Adrienne; Swash, M; Scott, DF (1972). "Effect of tyramine in migraine: a double-blind study". Journal of Neurology, Neurosurgery, & Psychiatry with Practical Neurology. 35, 4: 496–499. doi:10.1136/jnnp.35.4.496.
- ↑ D'Andrea, G; Nordera, GP; Perini, F; Allais, G; Granella, F (May 2007). "Biochemistry of neuromodulation in primary headaches: focus on anomalies of tyrosine metabolism". Neurological Sciences. 28, Supplement 2: S94–S96. doi:10.1007/s10072-007-0758-4. PMID 17508188
- ↑ Tyrosine metabolism - Reference pathway, Kyoto Encyclopedia of Genes and Genomes (KEGG)
- ↑ G. Barger (1909). J. Chem. Soc. 95: 1123.
- ↑ Waser, Ernst (1925). "Untersuchungen in der Phenylalanin-Reihe VI. Decarboxylierung des Tyrosins und des Leucins". Helvetica Chimica Acta 8: 758. doi:10.1002/hlca.192500801106.
- ↑ Buck, Johannes S. (1933). Journal of the American Chemical Society 55 (8): 3388. doi:10.1021/ja01335a058.
| |||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||||||||||