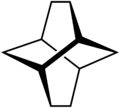
Twistane
| Twistane | ||
|---|---|---|
 |
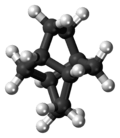 | |
| IUPAC name tricyclo[4.4.0.03,8]decane | ||
| Identifiers | ||
| CAS number | 253-14-5 | |
| Jmol-3D images | Image 1 | |
| ||
| ||
| Properties | ||
| Molecular formula | C10H16 | |
| Molar mass | 136.234 g/mol | |
| Melting point | 163-164.8°C[1] | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Twistane (IUPAC name: tricyclo[4.4.0.03,8]decane[2]) is an organic compound with the formula C10H16. It is a cycloalkane and an isomer of the simplest diamondoid, adamantane, and like adamantane, is not very volatile. Twistane was named for the way its rings are permanently forced into the cyclohexane conformation known as the "twist-boat".[1]
Synthesis
Twistane has been synthesized in a variety of ways. One path is by the intramolecular aldol condensation of a cis-decalin diketone. It is formed when basketane is hydrogenated.[3]
Symmetry
Although twistane has four stereocenters, it only exists as two enantiomers. This is because it is symmetric along its C2 axis.[4]
References
- ↑ 1.0 1.1 Beyer, Hans; Walter, Wolfgang; trans. Douglas Lloyd (1997), Organic Chemistry, Horwood Publishing, p. 416, ISBN 1-898563-37-3, retrieved 2008-12-09
- ↑ Quinkert, Gerhard; Egert, Ernst; Griesinger, Christian; trans. Andrew Beard (1996), Aspects of Organic Chemistry: Structure, Basel, Switzerland: Helvetica Chimica Acta, p. 107, ISBN 3-906390-15-2, retrieved 2008-12-09
- ↑ Ho, Tse-Lok (1995), Symmetry: A Basis for Synthesis Design, Wiley-IEEE, p. 69, ISBN 0-471-57376-0, retrieved 2008-12-10
- ↑ Kalsi, P. S. (2005), Stereochemistry Conformation and Mechanism, New Age Publishers, p. 94, ISBN 81-224-1564-4, retrieved 2008-12-10