Tulipanin
| Tulipanin | |
|---|---|
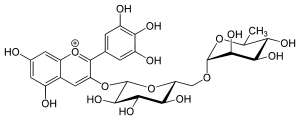 | |
| IUPAC name (2S,3R,4S,5S,6R)-2-[5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)chromenylium-3-yl]oxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxane-3,4,5-triol | |
| Other names Delphinidin-3-rutinoside | |
| Identifiers | |
| CAS number | 15674-58-5 |
| PubChem | 5492231 |
| ChemSpider | 4590910 |
| Jmol-3D images | {{#if:O(C[C@H]4O[C@@H](Oc2cc3c(O)cc(O)cc3[o+]c2c1cc(O)c(O)c(O)c1)[C@H](O)[C@@H](O)[C@@H]4O)[C@@H]5O[C@H]([C@H](O)[C@@H](O)[C@H]5O)C|Image 1 |
| |
| |
| Properties | |
| Molecular formula | C27H31ClO16 C27H31O16+ |
| Molar mass | 611.52 g/mol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Tulipanin is an anthocyanin. It is the 3-rutinoside of delphinidin. It can be found in Alstroemeria spp., Berberis spp., Cissus sicyoides, Hymenocallis spp., Manihot utilissima, Meliosma tenuis, Musa acuminata, Ophiopogon japonicus, Petunia exserta, Petunia reitzii, blackcurrant (Ribes nigrum), Schismatoglottis concinna, Secale cereale, Solanum betaceum, Thaumatococcus daniellii, Tulipa spp[1][2] and in eggplants.[3]
References
- ↑ Harborne, The Handbook of Natural Flavonoids, 2, (1999), 1, Anthocyanins
- ↑ Tulipanin on kanaya.naist.jp
- ↑ Structures and Antioxidant Activity of Anthocyanins in Many Accessions of Eggplant and Its Related Species. Keiko Azuma, Akio Ohyama, Katsunari Ippoushi, Takashi Ichiyanagi, Atsuko Takeuchi, Takeo Saito and Hiroyuki Fukuoka, J. Agric. Food Chem., 2008, 56 (21), pp 10154–10159, doi:10.1021/jf801322m
| ||||||||||||||||||||||||||||||||