Tryptamine
| Tryptamine | ||
|---|---|---|
 | ||
 | ||
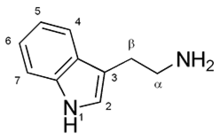
| IUPAC name 2-(1H-Indol-3-yl)ethanamine | ||
| Identifiers | ||
| CAS number | 61-54-1 | |
| PubChem | 1150 | |
| ChEMBL | CHEMBL6640 | |
| IUPHAR ligand | 125 | |
| Jmol-3D images | Image 1 | |
| ||
| ||
| Properties | ||
| Molecular formula | C10H12N2 | |
| Molar mass | 160.22 g mol−1 | |
| Appearance | white to orange crystalline powder[1] | |
| Melting point | 113-116˚C[1] | |
| Boiling point | 137˚C[1] | |
| Solubility in water | negligible solubility in water[1] | |
| Hazards | ||
| Flash point | 185˚C[1] | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | ||
| Infobox references | ||
Tryptamine is a monoamine alkaloid found in plants, fungi, and animals. It contains an indole ring structure, and is structurally similar to the amino acid tryptophan, from which it derives its name. Tryptamine is found in trace amounts in the brains of mammals and is believed to play a role as a neuromodulator or neurotransmitter.[2]
The tryptamine chemical structure is the backbone for a group of compounds termed collectively tryptamines. This group includes many biologically active compounds, including neurotransmitters and psychedelic drugs.
The concentration of tryptamine in rat brains is about 3.5 pmol/g.[3]
Plants containing tryptamine
Many plants contain small amounts of tryptamine, for example, as a possible intermediate in one biosynthetic pathway to the plant hormone indole-3-acetic acid.[4] Higher concentrations can be found in many Acacia species.
Role in vertebrates
Tryptamine acts as a serotonin releasing agent[5] and a serotonergic activity enhancer.[6] It is metabolised by MAO-A and MAO-B.[7]
Tryptamine derivatives
Well-known tryptamines include serotonin, an important neurotransmitter, and melatonin, a hormone involved in regulating the sleep-wake cycle. Tryptamine alkaloids found in fungi, plants and animals are sometimes used by humans and other animals, notably, the Jaguar, for their psychotropic effects. A Jaguar is seen eating yage (banisteriopsis caapi), a plant with high concentrations of the tryptamines harmine and harmaline, in an interesting clip borrowed from the "Peculiar Potions" episode of BBC's series "Weird Nature". Prominent examples of tryptamines include psilocybin (from "Psilocybin mushrooms") and DMT (from numerous plant sources, e.g. chacruna, often used in ayahuasca brews). Many synthetic tryptamines have also been made, including the migraine drug sumatriptan and its relatives. The tables below list some tryptamines.
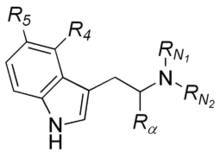
The tryptamine structure, in particular its indole ring, may be part of the structure of some more complex compounds, for example: LSD, ibogaine and yohimbine. A thorough investigation of dozens of tryptamine compounds was published by Ann and Alexander Shulgin under the title TiHKAL.
| Short Name | Origin | Rα | R4 | R5 | RN1 | RN2 | Full Name |
|---|---|---|---|---|---|---|---|
| Tryptamine | Natural | H | H | H | H | H | 3-(2-aminoethyl)indole / 2-(1H-indol-3-yl)ethanamine |
| Bufotenin | Natural | H | H | OH | CH3 | CH3 | 5-hydroxy-N,N-dimethyltryptamine |
| Nω-methylserotonin (norbufotenin) | Natural | H | H | OH | CH3 | H | 5-hydroxy-N-methyltryptamine |
| Serotonin | Natural | H | H | OH | H | H | 5-hydroxytryptamine |
| DMT | Natural | H | H | H | CH3 | CH3 | N,N-dimethyltryptamine |
| Melatonin | Natural | H | H | OCH3 | O=C-CH3 | H | 5-methoxy-N-acetyltryptamine |
| 5-Bromo-DMT | Natural | H | H | Br | CH3 | CH3 | 5-bromo-N,N-dimethyltryptamine |
| 5-MeO-DMT | Natural | H | H | OCH3 | CH3 | CH3 | 5-methoxy-N,N-dimethyltryptamine |
| 5-MeO-NMT | Natural | H | H | OCH3 | CH3 | H | 5-methoxy-N-methyltryptamine |
| NMT | Natural | H | H | H | H | CH3 | N-methyltryptamine |
| Norbaeocystin | Natural | H | OPO3H2 | H | H | H | 4-phosphoryloxy-tryptamine |
| Baeocystin | Natural | H | OPO3H2 | H | CH3 | H | 4-phosphoryloxy-N-methyl-tryptamine |
| Psilocybin | Natural | H | PO4 | H | CH3 | CH3 | 4-phosphoryloxy-N,N-dimethyltryptamine |
| Psilocin | Natural | H | OH | H | CH3 | CH3 | 4-hydroxy-N,N-dimethyltryptamine |
| Tryptophan | Natural | COOH | H | H | H | H | α-carboxyltryptamine |
| αET | artificial | CH2CH3 | H | H | H | H | α-ethyltryptamine |
| αMT | artificial | CH3 | H | H | H | H | α-methyltryptamine |
| DALT | artificial | H | H | H | H2C=CH-CH2 | H2C=CH-CH2 | N,N-diallyltryptamine |
| DET | artificial | H | H | H | CH2CH3 | CH2CH3 | N,N-diethyltryptamine |
| DiPT | artificial | H | H | H | CH(CH3)2 | CH(CH3)2 | N,N-diisopropyltryptamine |
| DPT | artificial | H | H | H | CH2CH2CH3 | CH2CH2CH3 | N,N-dipropyltryptamine |
| 5-MeO-αMT | artificial | CH3 | H | OCH3 | H | H | 5-methoxy-α-methyltryptamine |
| 5-MeO-DALT | artificial | H | H | OCH3 | H2C=CH-CH2 | H2C=CH-CH2 | 5-methoxy-N,N-diallyltryptamine |
| 4-HO-DET | artificial | H | OH | H | CH2CH3 | CH2CH3 | 4-hydroxy-N,N-diethyltryptamine |
| 4-AcO-DMT | artificial | H | OCOCH3 | H | CH3 | CH3 | 4-acetoxy-N,N-dimethyltryptamine |
| 4-HO-MET | artificial | H | OH | H | CH3 | CH2CH3 | 4-hydroxy-N-methyl-N-ethyltryptamine |
| 4-HO-DIPT | artificial | H | OH | H | CH(CH3)2 | CH(CH3)2 | 4-hydroxy-N,N-diisopropyltryptamine |
| 5-MeO-DIPT | artificial | H | H | OCH3 | CH(CH3)2 | CH(CH3)2 | 5-methoxy-N,N-diisopropyltryptamine |
| 4-HO-MiPT | artificial | H | OH | H | CH(CH3)2 | CH3 | 4-hydroxy-N-isopropyl-N-methyltryptamine |
| Sumatriptan | artificial | H | H | CH2SO2NHCH3 | CH3 | CH3 | 5-(methylaminosulfonylmethylene)-N,N-dimethyltryptamine |
| Zolmitriptan | artificial | H | H | -(CHNHC=OOCH2) | CH3 | CH3 | 5-( 4-(S)-1,3-oxazolidin-2-one)-N,N-dimethyltryptamine |
| Short Name | Origin | Rα | R4 | R5 | RN1 | RN2 | Full Name |
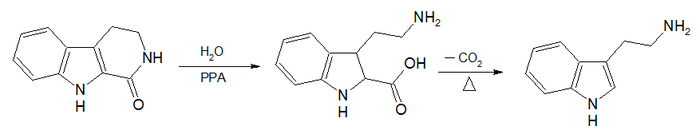
Synthesis
The Abramovitch–Shapiro tryptamine synthesis is an organic reaction for the synthesis of tryptamines starting from a β-carboline.[8]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "http://www.chemicalbook.com/ProductChemicalPropertiesCB8192006_EN.htm"
- ↑ Jones R.S. (1982). "Tryptamine: a neuromodulator or neurotransmitter in mammalian brain?". Progress in neurobiology 19 (1–2): 117–139. doi:10.1016/0301-0082(82)90023-5.
- ↑ Jiang, Zhen; Mutch, Elaine; Blain, Peter G.; Williams, Faith M. (2006). "Conversion of trichloroethylene to chloral using occupationally relevant levels". Toxicology 226: 76. doi:10.1016/j.tox.2006.05.102.
- ↑ Nobutaka Takahashi (1986). Chemistry of Plant Hormones. CRC Press. ISBN 9780849354700.
- ↑ Wölfel, Reinhard; Graefe, Karl-Heinz (1992). "Evidence for various tryptamines and related compounds acting as substrates of the platelet 5-hydroxytryptamine transporter". Naunyn-Schmiedeberg's Archives of Pharmacology 345 (2): 129–36. doi:10.1007/BF00165727. PMID 1570019.
- ↑ Shimazu, S; Miklya, I (2004). "Pharmacological studies with endogenous enhancer substances: Beta-phenylethylamine, tryptamine, and their synthetic derivatives". Progress in neuro-psychopharmacology & biological psychiatry 28 (3): 421–7. doi:10.1016/j.pnpbp.2003.11.016. PMID 15093948.
- ↑ Sullivan, James P.; McDonnell, Leonard; Hardiman, Orla M.; Farrell, Michael A.; Phillips, Jack P.; Tipton, Keith F. (1986). "The oxidation of tryptamine by the two forms of monoamine oxidase in human tissues". Biochemical Pharmacology 35 (19): 3255–60. doi:10.1016/0006-2952(86)90421-1. PMID 3094536.
- ↑ Abramovitch, R. A.; Shapiro, D. (1956). "880. Tryptamines, carbolines, and related compounds. Part II. A convenient synthesis of tryptamines and ?-carbolines". Journal of the Chemical Society (Resumed): 4589. doi:10.1039/JR9560004589.
External links
- Tryptamine FAQ
- Tryptamine Hallucinogens and Consciousness
- Tryptamind Psychoactives, reference site on tryptamine and other psychoactives.
- Tryptamine (T) entry in TiHKAL • info
| |||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||
| |||||