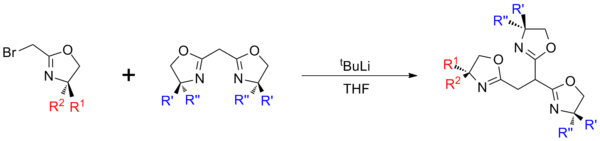Trisoxazolines
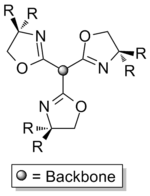
Trisoxazolines (Often abbreviated TRISOX or TOX) are a class of tridentate, chiral ligands composed of three oxazoline rings. Despite being neutral they are able to form stable complexes with high oxidation state metals, such as rare earths,[1] due to the chelate effect. The ligands have been investigated for molecular recognition and their complexes are used in asymmetric catalysts and polymerisation.
Synthesis
Trisoxazolines can either be synthesised directly, from suitable tripodal starting materials, or built up in a modular manner.[2] These approaches can be used to give ligands of differing symmetries, with the direct synthesis route giving homochiral ligands with C3 rotational symmetry and the modular approach typically being used to give asymmetric compounds (C1 symmetry), which are either heterochiral or possess a mix of both chiral and achiral groups. These differences in symmetry can significantly effect the coordination chemistry of the ligands[3] and the catalytic activity of their complexes, with C3 symmetric ligands often being better for asymmetric catalysis.[4][5]
Direct methods
Suitable tripodal compounds, such as trimesic acid[6] and nitrilotriacetic acid,[7] can be converted directly to trisoxazolines. The simplicity of this approach is beneficial, however it only allows a limited variety of structures to be produced, due to the limited range of available starting materials.
Modular methods
Modular synthesis allows for a more diverse range of structures, however the multi-step reactions can result in lower overall yields. In general synthesis involves the generation of separate mono‑oxazoline (typically halogenated) and bis-oxazoline units, which are then coupled using a strong base such as tBuLi[8] or KN(SiMe3)2.
In addition to the inclusion of heterochirality, modular synthesis also allows for the synthesis of 'lopsided' structures which have application as scorpionate ligands.[9]
In catalysis
Friedel–Crafts reaction
Trisoxazolines have been used for the copper catalysed Friedel–Crafts alkylation of indoles, largely with alkylidene malonates, with good yields and ee's reported. A number of interesting solvent effects have also been observed, including a relationship between enantioselectivity and the steric bulk of the solvent when using of alcohols[10] and a reversal of enantioselectivity when changing the reaction solvent from coordinating solvents to weakly coordinating solvents.[11]
Polymerisation
Rare earth complexes incorporating TRISOX ligands have been found to be highly effective catalysts for the polymerisation of α-alkenes and are notable for producing polyolefins with very high tacticities.[12][13] Computational modelling of the polymerisation mechanism indicates that kinetic factors likely account for the high tactisity.[14]
Molecular recognition
Trisoxazolines baring a benzene backbone have been investigated for molecular recognition and have shown promising selectivity for the recognition of ammonium[15] alkylammonium and sugar species,[16] including examples of chiral recognition.
See also
References
- ↑ Ward, Benjamin D.; Gade, Lutz H. (1 January 2012). "Rare earth metal oxazoline complexes in asymmetric catalysis". Chemical Communications 48 (86): 10587. doi:10.1039/c2cc34997c.
- ↑ Zhou, Jian; Tang, Yong (1 January 2005). "The development and application of chiral trisoxazolines in asymmetric catalysis and molecular recognition". Chemical Society Reviews 34 (8): 664. doi:10.1039/B408712G.
- ↑ Gade, Lutz H.; Marconi, Guido; Dro, Clémence; Ward, Benjamin D.; Poyatos, Macarena; Bellemin-Laponnaz, Stéphane; Wadepohl, Hubert; Sorace, Lorenzo; Poneti, Giordano. "Shaping and Enforcing Coordination Spheres: The Implications of C3 andC1 Chirality in the Coordination Chemistry of 1,1,1-Tris(oxazolinyl)ethane ("Trisox")". Chemistry - A European Journal 13 (11): 3058–3075. doi:10.1002/chem.200601651.
- ↑ Gade, Lutz H.; Bellemin-Laponnaz, Stéphane. "Exploiting Threefold Symmetry in Asymmetric Catalysis: The Case of Tris(oxazolinyl)ethanes ("Trisox")". Chemistry - A European Journal 14 (14): 4142–4152. doi:10.1002/chem.200701990.
- ↑ Foltz, Carole; Stecker, Björn; Marconi, Guido; Bellemin-Laponnaz, Stéphane; Wadepohl, Hubert; Gade, Lutz H. (23 October 2007). "Stereochemical Consequences of Threefold Symmetry in Asymmetric Catalysis: Distorting C3 Chiral 1,1,1-Tris(oxazolinyl)ethanes ("Trisox") in CuII Lewis Acid Catalysts". Chemistry - A European Journal 13 (35): 9912–9923. doi:10.1002/chem.200701085.
- ↑ Kim, Hae-Jo; Kim, Yeon-Hwan; Hong, Jong-In. "Sugar recognition by C3-symmetric oxazoline hosts". Tetrahedron Letters 42 (30): 5049–5052. doi:10.1016/S0040-4039(01)00915-7.
- ↑ Kawasaki, Ken-ichi; Katsuki, Tsutomu. "Enantioselective allylic oxidation of cycloalkenes by using Cu(II)-tris(oxazoline) complex as a catalyst". Tetrahedron 53 (18): 6337–6350. doi:10.1016/S0040-4020(97)00322-0.
- ↑ Bellemin-Laponnaz, Stéphane; Gade, Lutz H. "A Modular Approach to C1 and C3 Chiral N-Tripodal Ligands for Asymmetric Catalysis". Angewandte Chemie International Edition 41 (18): 3473–3475. doi:10.1002/1521-3773(20020916)41:18<3473::AID-ANIE3473>3.0.CO;2-N.
- ↑ Ye, Meng-Chun; Li, Bin; Zhou, Jian; Sun, Xiu-Li; Tang, Yong (1 July 2005). "Modular Synthesis of Chiral Homo- and Heterotrisoxazolines: Improving the Enantioselectivity in the Asymmetric Michael Addition of Indole to Benzylidene Malonate". The Journal of Organic Chemistry 70 (15): 6108–6110. doi:10.1021/jo050595m.
- ↑ Zhou, Jian; Ye, Meng-Chun; Huang, Zheng-Zheng; Tang, Yong (1 February 2004). "Controllable Enantioselective Friedel−Crafts Reaction between Indoles and Alkylidene Malonates Catalyzed by Pseudo-C3-Symmetric Trisoxazoline Copper(II) Complexes". The Journal of Organic Chemistry 69 (4): 1309–1320. doi:10.1021/jo035552p.
- ↑ Zanoni, Giuseppe; Castronovo, Francesca; Franzini, Maurizio; Vidari, Giovanni; Giannini, Elios. "Toggling enantioselective catalysis—a promising paradigm in the development of more efficient and versatile enantioselective synthetic methodologies". Chemical Society Reviews 32 (3): 115–129. doi:10.1039/B201455F.
- ↑ Lukešová, Lenka; Ward, Benjamin D.; Bellemin-Laponnaz, Stéphane; Wadepohl, Hubert; Gade, Lutz H. (1 January 2007). "High tacticity control in organolanthanide polymerization catalysis: formation of isotactic poly(α-alkenes) with a chiral C3-symmetric thulium complex". Dalton Transactions (9): 920. doi:10.1039/B700269F.
- ↑ Ward, Benjamin D.; Lukešová, Lenka; Wadepohl, Hubert; Bellemin-Laponnaz, Stéphane; Gade, Lutz H. (1 March 2009). "Scandium-Catalyzed Polymerization of CH3(CH2)nCH=CH2 (n = 0–4): Remarkable Activity and Tacticity Control". European Journal of Inorganic Chemistry 2009 (7): 866–871. doi:10.1002/ejic.200801106.
- ↑ Kang, Xiaohui; Song, Yuming; Luo, Yi; Li, Gang; Hou, Zhaomin; Qu, Jingping. "Computational Studies on Isospecific Polymerization of 1-Hexene Catalyzed by Cationic Rare Earth Metal Alkyl Complex Bearing a Pr-trisox Ligand". Macromolecules 45 (2): 640–651. doi:10.1021/ma202414k.
- ↑ Kim, Sung-Gon; Kim, Kyung-Hyun; Jung, Junyang; Shin, Seung Koo; Ahn, Kyo Han (1 January 2002). "Unprecedented Chiral Molecular Recognition in a C3-Symmetric Environment". Journal of the American Chemical Society 124 (4): 591–596. doi:10.1021/ja0119696.
- ↑ Kim, Hae-Jo; Kim, Yeon-Hwan; Hong, Jong-In. "Sugar recognition by C3-symmetric oxazoline hosts". Tetrahedron Letters 42 (30): 5049–5052. doi:10.1016/S0040-4039(01)00915-7.