Trimethylarsine
| Trimethylarsine | |
|---|---|
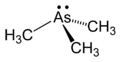 |
 |
| Trimethanidoarsenic | |
| Systematic name Trimethylarsane | |
| Other names Gosio gas | |
| Identifiers | |
| CAS number | 593-88-4 |
| PubChem | 68978 |
| ChemSpider | 62200 |
| EC number | 209-815-8 |
| MeSH | Trimethylarsine |
| ChEBI | CHEBI:27130 |
| RTECS number | CH8800000 |
| Beilstein Reference | 1730780 |
| Gmelin Reference | 141657 |
| Jmol-3D images | {{#if:[As](C)(C)CC[As](C)C|Image 1 Image 2 |
| |
| |
| Properties | |
| Molecular formula | C3H9As |
| Molar mass | 120.03 g mol−1 |
| Appearance | Colourless liquid |
| Density | 1.124 g cm-3 |
| Melting point | −87.3 °C; −125.1 °F; 185.8 K |
| Boiling point | 56 °C; 133 °F; 329 K |
| Solubility in water | Slightly soluble |
| Solubility in other solvents | organic solvents |
| Structure | |
| Coordination geometry |
Trigonal pyramidal |
| Dipole moment | 0.86 D |
| Hazards | |
| MSDS | External MSDS |
| R-phrases | R23/25 R50/53 |
| S-phrases | (S1/2) S20/21 S28 S45 S60 S61 |
| Main hazards | Flammable |
| Flash point | −25 °C; −13 °F; 248 K |
| Related compounds | |
| Related compounds | Cacodylic acid Triphenylarsine |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C (77 °F), 100 kPa) | |
| Infobox references | |
Trimethylarsine is the chemical compound with the formula (CH3)3As, commonly abbreviated AsMe3 or TMAs. This organic derivative of arsine has been used as a source of arsenic in microelectronics industry,[1] a building block to other organoarsenic compounds, and serves as a ligand in coordination chemistry. It has distinct "garlic"-like smell. Trimethylarsine had been discovered as early as 1854.
Structure and preparation
As predicted by VSEPR theory, AsMe3 is a pyramidal molecule. The As-C distances average 1.519 Å, and the C-As-C angles are 91.83°[2] This bond angle is a strong indication of a low, if any, hybridisation of the atomic orbitals, leaving the lone pair in the s-orbital buried in the inner regions of the arsenic atom, rather than pointing outward like the lone pair of the ammonia molecule.
Trimethylarsine can be prepared by treatment of arsenic oxide with trimethylaluminium:[3]
- As2O3 + 1.5 [AlMe3]2 → 2 AsMe3 + 3/n (MeAl-O)n
Properties and reactions
Trimethylarsine is pyrophoric due to the exothermic nature of the following reaction, which initiates combustion:
- AsMe3 + 1/2 O2 → OAsMe3 (TMAO)
History
Poisoning events due to a gas produced by certain microbes was assumed to be associated with the arsenic in paint. In 1893 the Italian physician Bartolomeo Gosio published his results on "Gosio gas" that was subsequently shown to contain trimethylarsine.[4] Under wet conditions, the mold Scopulariopsis brevicaulis produces significant amounts of methyl arsines via methylation[5] of arsenic-containing inorganic pigments, especially Paris green and Scheele's Green, which were once used in indoor wallpapers. Newer studies show that trimethylearsine has a low toxicity and could therefore not account for the death and the severe health problems observed in the 19th century.[6] [7]
Safety
Trimethylarsine is potentially hazardous,[8][9][10] although its toxicity is often overstated.[6]
Importance
Trimethylarsine is the volatile byproduct of microbial action on inorganic forms of arsenic which are naturally occurring in rocks and soils at the parts-per-million level.[11] Trimethylarsine has been reported only at trace levels (parts per billion) in landfill gas from Germany, Canada, and the U.S.A., and is the major arsenic-containing compound in the gas.[12][13][14]
References
- ↑ Hoshino, Masataka (1991). "A mass spectrometric study of the decomposition of trimethylarsine (TMAs) with triethylgallium (TEGa)". Journal of Crystal Growth 110 (4): 704–712. doi:10.1016/0022-0248(91)90627-H.
- ↑ Wells, A.F. (1984). Structural Inorganic Chemistry, fifth edition. Oxford University Press. ISBN 0-19-855370-6.
- ↑ V. V. Gavrilenko, L. A. Chekulaeva, and I. V. Pisareva, "Highly efficient synthesis of trimethylarsine" Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 8, pp. 2122–2123, 1996.
- ↑ Frederick Challenger (1955). "Biological methylation". Q. Rev. Chem. Soc. 9 (3): 255–286. doi:10.1039/QR9550900255.
- ↑ Ronald Bentley and Thomas G. Chasteen (2002). "Microbial Methylation of Metalloids: Arsenic, Antimony, and Bismuth". Microbiology and Molecular Biology Reviews 66 (2): 250–271. doi:10.1128/MMBR.66.2.250-271.2002. PMC 120786. PMID 12040126.
- ↑ 6.0 6.1 William R. Cullen, Ronald Bentley (2005). "The toxicity of trimethylarsine: an urban myth". J. Environ. Monit. 7 (1): 11–15. doi:10.1039/b413752n. PMID 15693178.
- ↑ Frederick Challenger, Constance Higginbottom, Louis Ellis (1933). "The formation of organo-metalloidal compounds by microorganisms. Part I. Trimethylarsine and dimethylethylarsine". J. Chem. Soc.: 95–101. doi:10.1039/JR9330000095.
- ↑ Andrewes, Paul, et al. (2003). "Dimethylarsine and Trimethylarsine Are Potent Genotoxins In Vitro". Chem. Res. Toxicol. 16 (8): 994–1003.
- ↑ Irvin, T.Rick, et al. (1995). "In-vitro Prenatal Toxicity of Trimethylarsine, Trimethylarsine Oxide and Trimethylarsine Sulfide". Applied Organometallic Chemistry 9: 315–321.
- ↑ Hiroshi Yamauchi, Toshikazu Kaise, Keiko Takahashi, Yukio Yamamura (1990). "Toxicity and metabolism of trimethylarsine in mice and hamsters". Fundamental and Applied Toxicology 14 (2): 399–407. doi:10.1016/0272-0590(90)90219-A. PMID 2318361.
- ↑ Cullen, W.R., Reimer, K.J. (1989). "Arsenic speciation in the environment". Chem. Reviews 89 (4): 713–764,. doi:10.1021/cr00094a002.
- ↑ Feldmann, J., Cullen, W.R. (1997). "Occurrence of Volatile Transition Metal Compounds in Landfill Gas: Synthesis of Molybdenum and Tungsten Carbonyls in the". Environ. Sci. Technol. 31 (7): 2125–2129. doi:10.1021/es960952y.
- ↑ Pinel-Raffaitin, P., LeHecho, I., Amouroux, D., Potin-Gautier, M. (2007). "Distribution and Fate of Inorganic and Organic Arsenic Species in Landfill Leachates and Biogases". Environ. Sci. Technol. 41 (13): 4536–4541. doi:10.1021/es0628506. PMID 17695893.
- ↑ Khoury, J.T. et al. (April 7, 2008). "Analysis of Volatile Arsenic Compounds in Landfill Gas". Odors & Air Emissions 2008. Phoenix, Arizona: Water Environment Federation.
External links
- Index by Molecular Formula
- Information on Hazardous Chemicals by Class
- Microbial Methylation of Metalloids: Arsenic, Antimony, and Bismuth
- Arsenic Curiosa and Humanity